Question: Part A Calculate AHEX for this reaction using standard enthalpies of formation. (The standard enthalpy of formation of liquid benzene is 49.1 kJ/mol.) Express the
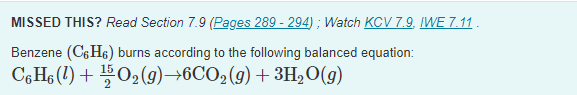
Part A Calculate AHEX for this reaction using standard enthalpies of formation. (The standard enthalpy of formation of liquid benzene is 49.1 kJ/mol.) Express the enthalpy in kilojoules to four significant figures. View Available Hint(s) ? . 11 kJ Submit MISSED THIS? Read Section 7.9 (Pages 289-294); Watch KCV 7.9. IWE 7.11. Benzene (CfH6) burns according to the following balanced equation: C.H6 (1) + 102(9)6C02 (9) + 3H,0(9)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


