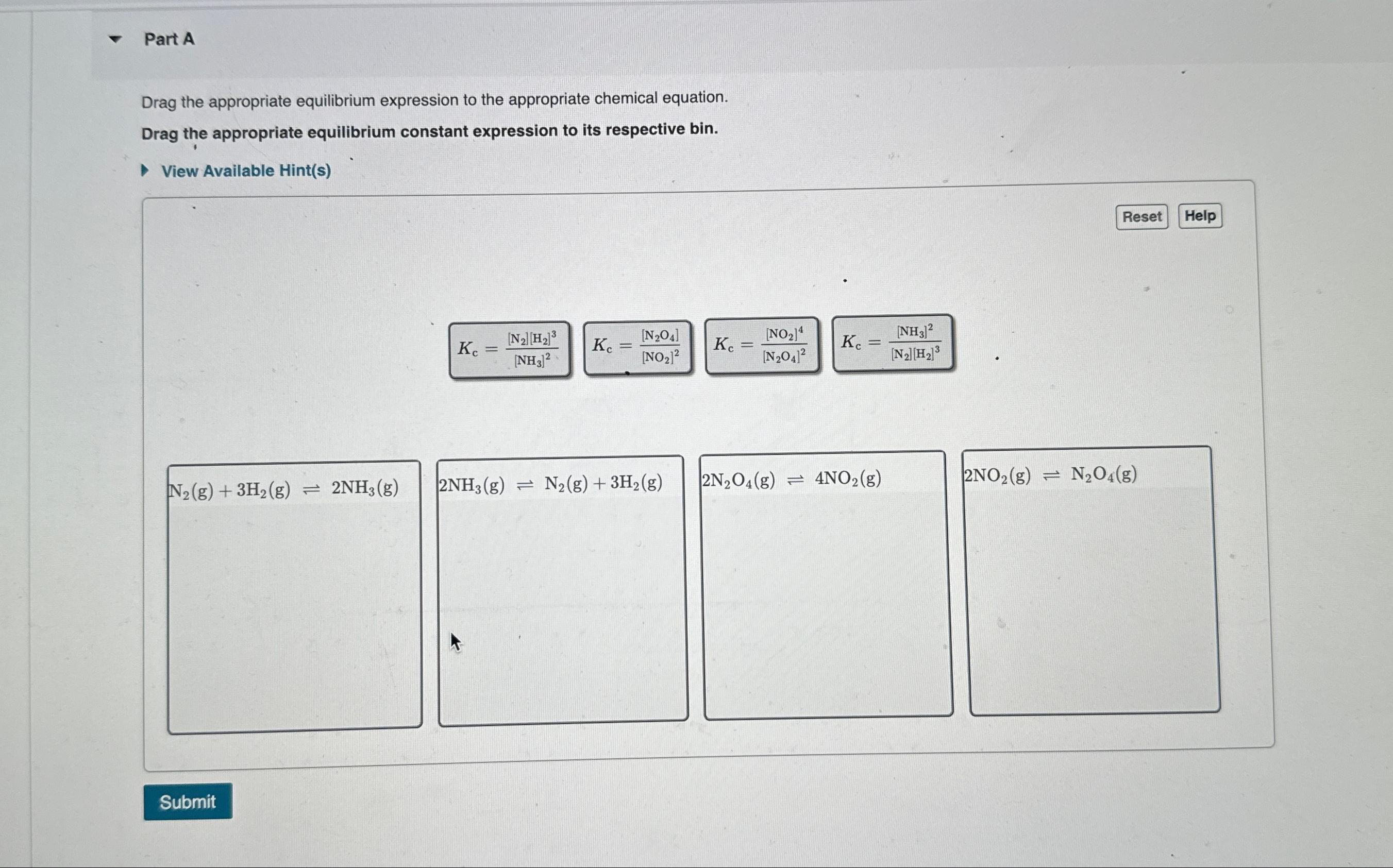
Question: Part A Drag the appropriate equilibrium expression to the appropriate chemical equation. Drag the appropriate equilibrium constant expression to its respective bin. View Available Hint
Part A
Drag the appropriate equilibrium expression to the appropriate chemical equation.
Drag the appropriate equilibrium constant expression to its respective bin.
View Available Hints
Reset
Help
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


