Question: Part A MISSED THIS? Read Section 14.6 (Pages 601 - 613); Watch KCV 14.6. IWE 14.9. Calculate the freezing point and boiling point of a
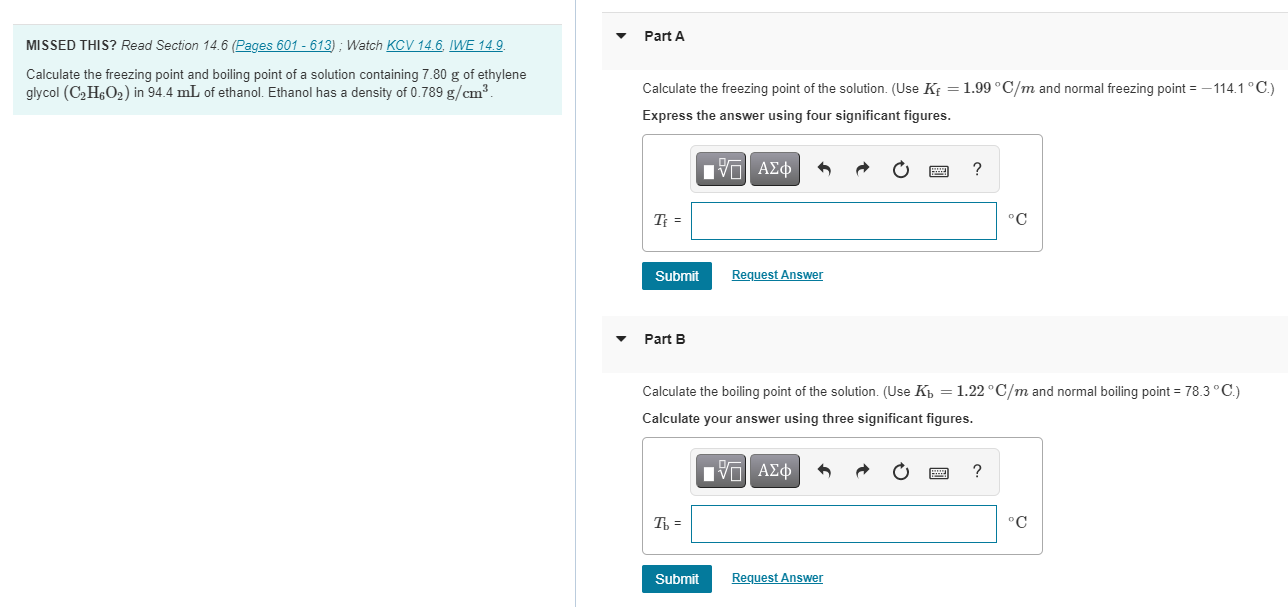
Part A MISSED THIS? Read Section 14.6 (Pages 601 - 613); Watch KCV 14.6. IWE 14.9. Calculate the freezing point and boiling point of a solution containing 7.80 g of ethylene glycol (C2H2O2) in 94.4 mL of ethanol. Ethanol has a density of 0.789 g/cm? Calculate the freezing point of the solution. (Use Kf = 1.99C/m and normal freezing point = -114.1 C.) Express the answer using four significant figures. ? TE = C Submit Request Answer Part B Calculate the boiling point of the solution. (Use K = 1.22C/m and normal boiling point = 78.3C.) Calculate your answer using three significant figures. VO AO ? T = C Submit Request
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


