Question: part a part b A buffer solution is 0.475M in HF and 0.316M in NaF. If Kn for HF is 7.2104, what is the pH

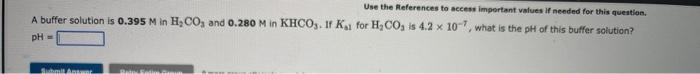
A buffer solution is 0.475M in HF and 0.316M in NaF. If Kn for HF is 7.2104, what is the pH of this buffer solution? pH= Use the Heferences to aceess important values if needed for this queatlon. A buffer solution is 0.395M in H2CO3 and 0.280M in KHCO3. If Kal for H2CO3 is 4.2107, what is the pH of this buffer solution? pH=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


