Question: part a part b A buffer solution made from HF and NaF has a pH of 2.94, If pKs for HF is 3.14, what is

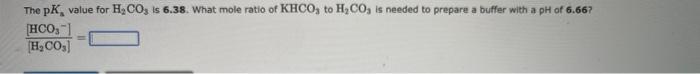
A buffer solution made from HF and NaF has a pH of 2.94, If pKs for HF is 3.14, what is the [HFF][F] in the buffer? [HF][F]= The pKa value for H2CO3 is 6.38. What mole ratio of KHCO3 to H2CO3 is needed to prepare a buffer with a pH of 6.66? [H2CO3][HCO3]=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


