Question: Part A The Arthenius equation shows the relationship between the rate constant k and the temperature Tin kelvins and is typically written as k=Ae-/ The
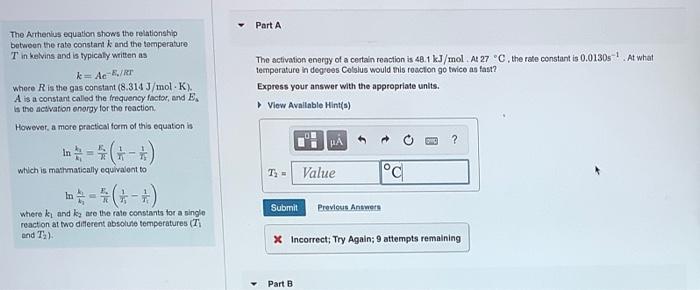

Part A The Arthenius equation shows the relationship between the rate constant k and the temperature Tin kelvins and is typically written as k=Ae-/ The activation energy of a certain reaction is 48.1 kJ/mol At 27 C, the rate constant is 0.0130s! At what temperaturo in degrees Celsius would this reaction go twice as fast? Express your answer with the appropriate units. View Available Hint(s) where is the gas constant (8.314 J/mol K). A is a constant called the frequency factor, and E. is the activation energy for the reaction However, a more practical form of this equation is In - ( which is mathmatically equivalent to p 1) - Value 1C Submit Previous Answers where ku and ky are the rate constants for a single reaction at two different absoluto temperatures (T and T) X Incorrect; Try Again; 9 attempts remaining Part B Part B a single uros (T) Given that the initial rate constant is 0.0130s at an initial temperature of 27 C, what would the rate constant be at a temperature of 180C for the same reaction described in Part A? Express your answer with the appropriate units. View Avallable Hint(s) ? k Value Units Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


