Question: Part A The rigid rotor model can be improved by recognizing that in a realistic anharmonic potential, the bond length increases with the vibrational quantum
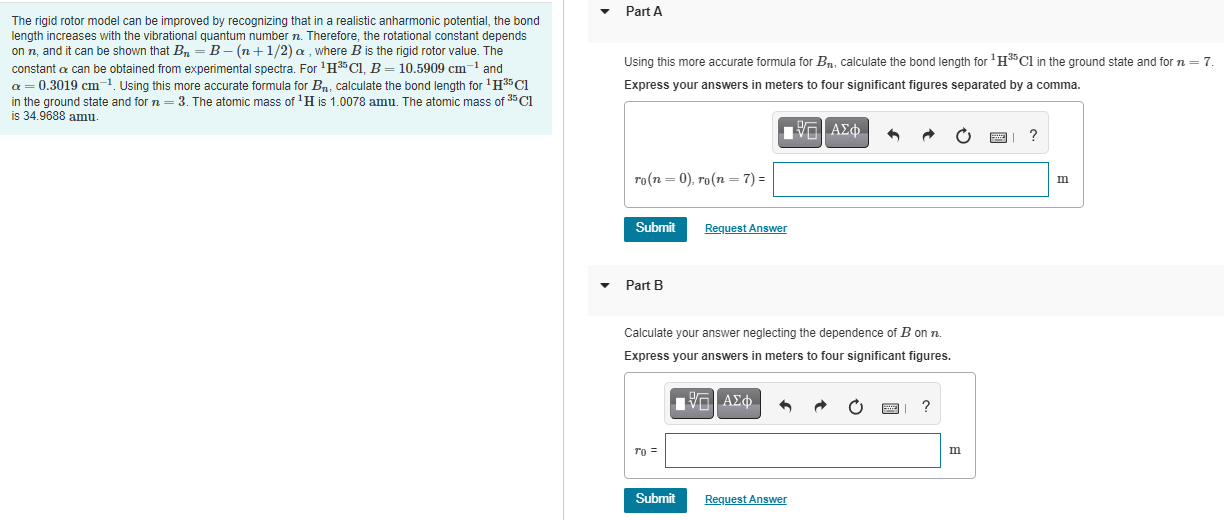
Part A The rigid rotor model can be improved by recognizing that in a realistic anharmonic potential, the bond length increases with the vibrational quantum number n. Therefore, the rotational constant depends on n, and it can be shown that Br=B-(n+1/2) a where B is the rigid rotor value. The constant a can be obtained from experimental spectra. For 135CI, B= 10.5909 cm 1 and a=0.3019 cm-1. Using this more accurate formula for Br, calculate the bond length for ''CI in the ground state and for n=3. The atomic mass of H is 1.0078 amu. The atomic mass of 35 CI is 34.9688 amu. Using this more accurate formula for Br, calculate the bond length for HCl in the ground state and for n=7. Express your answers meters to four significant figures separated by a comma. TVO AKO ? ro(n=0), ro(n=7) = m Submit Request Answer Part B Calculate your answer neglecting the dependence of Bonn Express your answers meters to four significant figures. EVO AKO ? To = m Submit Request
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


