Question: Part A - Translate the formulaic equations to word equations. 1. Na2O + H2O ---> NaOH 2.2HgO(s) ---> 2Hg() + O2(g) 3. BaCl2(aq) + Na2CrO4(aq)
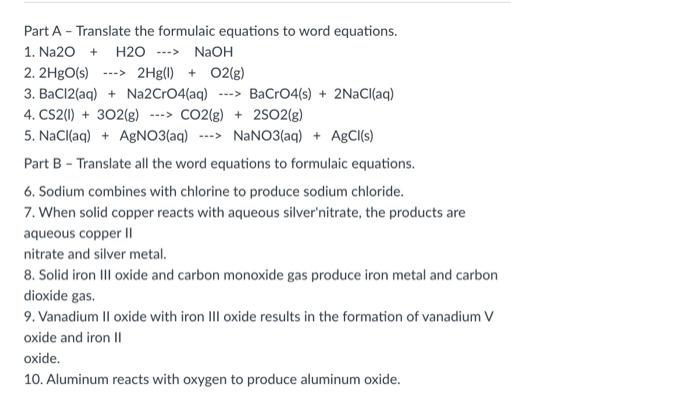
Part A - Translate the formulaic equations to word equations. 1. Na2O + H2O ---> NaOH 2.2HgO(s) ---> 2Hg() + O2(g) 3. BaCl2(aq) + Na2CrO4(aq) ---> BaCrO4(s) + 2NaCl(aq) 4. CS2(1) + 302(g) ---> CO2(g) + 2502(g) 5. NaCl(aq) + AgNO3(aq) ---> NaNO3(aq) + AgCl(s) Part B - Translate all the word equations to formulaic equations. 6. Sodium combines with chlorine to produce sodium chloride. 7. When solid copper reacts with aqueous silver'nitrate, the products are aqueous copper 11 nitrate and silver metal. 8. Solid iron Ill oxide and carbon monoxide gas produce iron metal and carbon dioxide gas, 9. Vanadium II oxide with iron III oxide results in the formation of vanadium V oxide and iron 11 oxide. 10. Aluminum reacts with oxygen to produce aluminum oxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


