Question: Part A Use the data below to calculate the heat of hydration of lithium chloride. Express the answer in kilojoules per mole using three
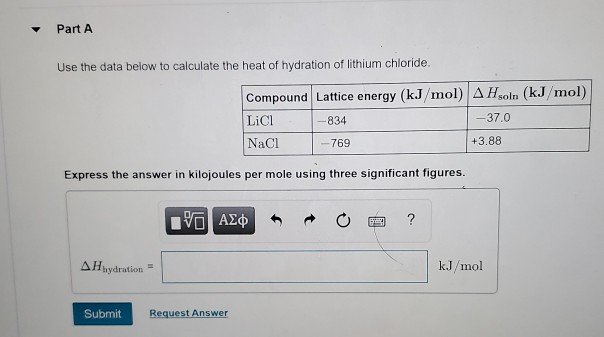

Part A Use the data below to calculate the heat of hydration of lithium chloride. Express the answer in kilojoules per mole using three significant figures. AHhydration Submit Compound Lattice energy (kJ/mol) A Hsoln (kJ/mol) LiCl -834 -37.0 NaCl -769 15. Request Answer P ? +3.88 kJ/mol Part B Calculate the heat of hydration of sodium chloride. Express the answer in kilojoules per mole using three significant figures. AHhydration Submit [ VO Request Answer ? kJ/mol Part C Identify the best explanation for which cation, lithium or sodium, has stronger ion-dipole interactions with water and why. O Since AHhydration of NaCl is more negative than for LiCl, NaCl has the stronger ion-dipole interactions. O Since A Hydration of NaCl is more positive than for LiCl, NaCl has the stronger ion-dipole interactions. Since Hydration of LiCl is more negative than for NaCl, LiCl has the stronger ion-dipole interactions. O Since AHydration of LiCl is more positive than for NaCl, LiCl has the stronger ion-dipole interactions. Submit Request Answer Next >
Step by Step Solution
3.48 Rating (158 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


