Question: part B and C please Estimate the molar solubility, S, of BaF2 in pure water given that the Ksp of BaF2 is 2.45105 View Available
part B and C please 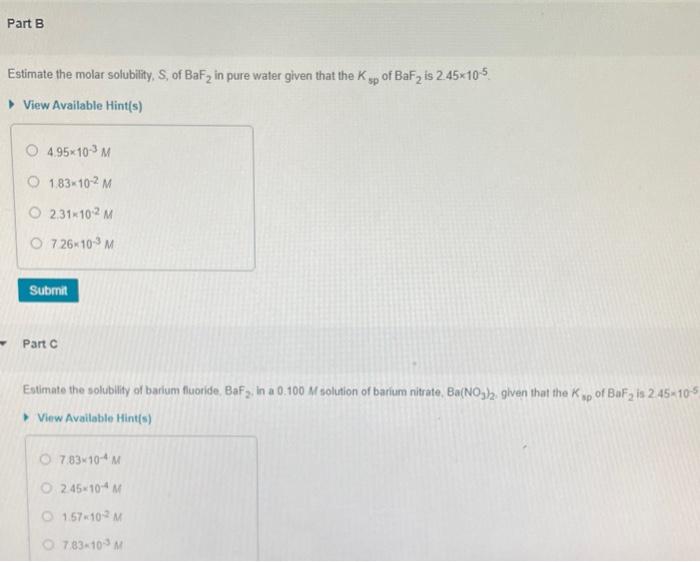

Estimate the molar solubility, S, of BaF2 in pure water given that the Ksp of BaF2 is 2.45105 View Available Hint(s) 4.95103M1.83102M2.31102M7.26103M Part C Estimate the solubility of barium fluoride, BaF2, in a 0.100M solution of barium nitrate. Ba(NO N3, given that the Ksp of BaF2 is 2.45105 View Available Hint(s) 7.83104M2.45104M1.57102M7.83103M
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


