Question: Part B: Sometimes more than one atom arrangement is possible; these are called geometrical isomers. Complete the steps for the following list of molecules: SF4
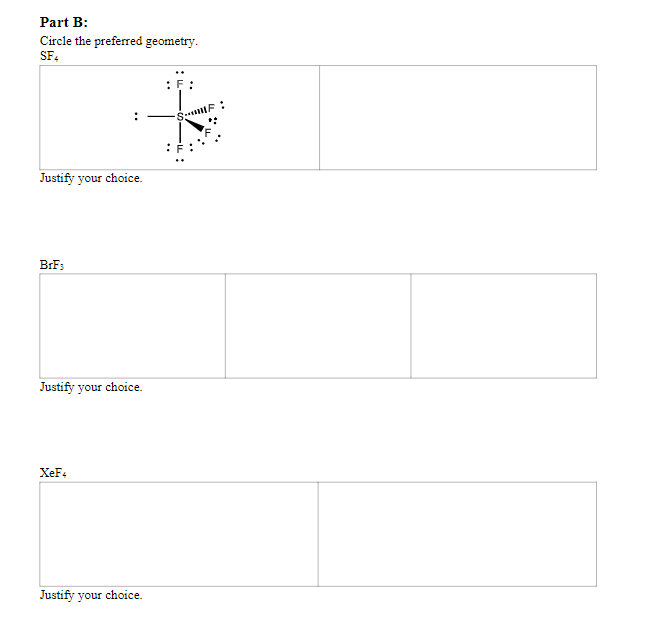
Part B: Sometimes more than one atom arrangement is possible; these are called geometrical isomers. Complete the steps for the following list of molecules: SF4 (2 isomers), BrF3 (3 isomers), XeF4 (2 isomers) 1. Sketch all the possible geometric isomers. 2. Then, keeping in mind that non-bonding pairs on the central atom will try to get as far away as possible from both other bonding and nonbonding pairs, predict which will be the preferred geometry in nature. Justify your choice. 3. Name the molecular geometry of the preferred structure.
Part B: Circle the preferred geometry. SF4
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


