Question: Part B - The pH Concept (possible 14 points) If you have taken Chemistry, you may recall molarity or molar concentration. Molarity is the number
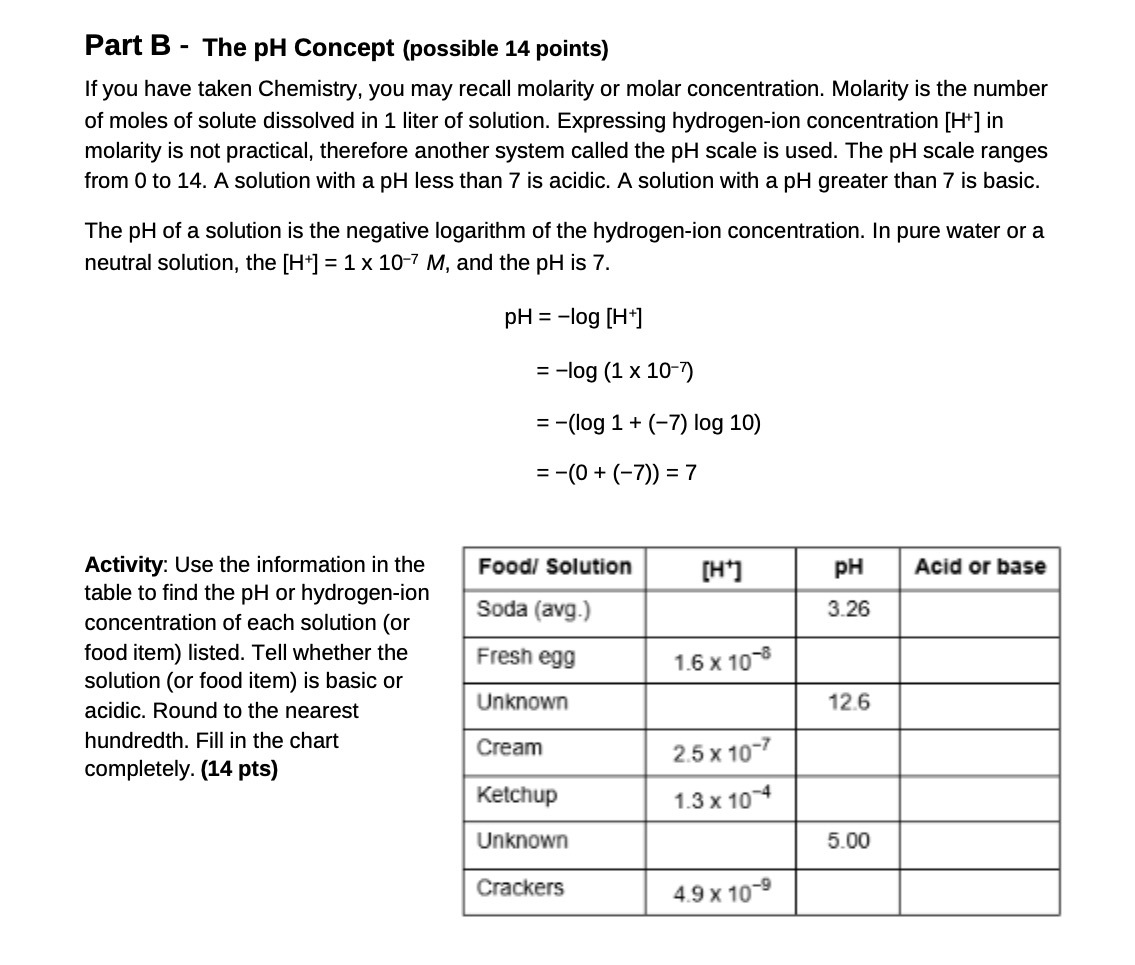
Part B - The pH Concept (possible 14 points) If you have taken Chemistry, you may recall molarity or molar concentration. Molarity is the number of moles of solute dissolved in 1 liter of solution. Expressing hydrogen-ion concentration [H* ] in molarity is not practical, therefore another system called the pH scale is used. The pH scale ranges from 0 to 14. A solution with a pH less than 7 is acidic. A solution with a pH greater than 7 is basic. The pH of a solution is the negative logarithm of the hydrogen-ion concentration. In pure water or a neutral solution, the [H*] = 1 x 10-7 M, and the pH is 7. PH = -log [H+] = -log (1 x 10-7) = -(log 1 + (-7) log 10) = -(0 + (-7)) = 7 Activity: Use the information in the Food/ Solution [H+] PH Acid or base table to find the pH or hydrogen-ion concentration of each solution (or Soda (avg.) 3.26 food item) listed. Tell whether the Fresh egg 1.6 x 10-8 solution (or food item) is basic or acidic. Round to the nearest Unknown 12.6 hundredth. Fill in the chart Cream 2.5 x 10- completely. (14 pts) Ketchup 1.3 x 10 4 Unknown 5.00 Crackers 4.9 x 10-9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


