Question: Part C Consider the reaction 5 B r - ( a q ) + B r O 3 - ( a q ) + 6
Part C
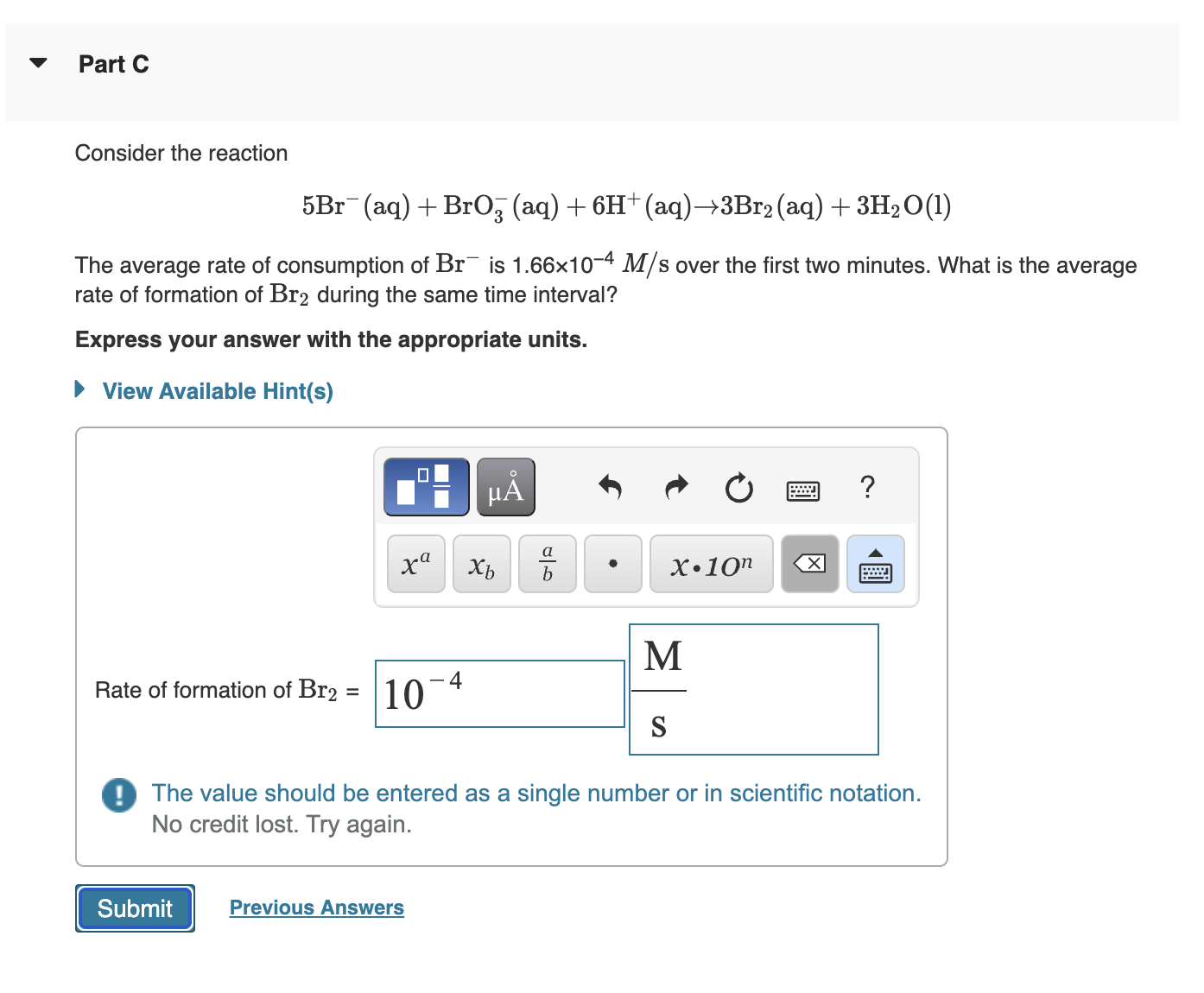
Consider the reaction
The average rate of consumption of is over the first two minutes. What is the average
rate of formation of during the same time interval?
Express your answer with the appropriate units.
View Available Hints
Rate of formation of
The value should be entered as a single number or in scientific notation.
ANSWER AS SINGLE NUMBER OR SCIENTIFIC NOTATION
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


