Question: Part I: Sketch a voltaic cell made with a Copper and Zinc strip with .002M Zinc sulphate and Copper II sulphate solutions. ( you will

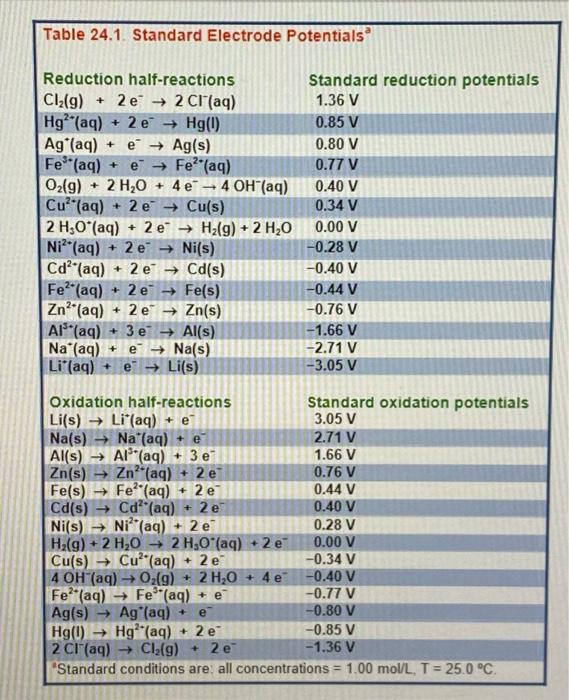
Part I: Sketch a voltaic cell made with a Copper and Zinc strip with .002M Zinc sulphate and Copper II sulphate solutions. ( you will need to use Table 24.1 on page 9chpt24 ) /3 a) Will oxidation occur at the Zinc or Aluminum electrode? /1 b) Write the reduction reaction. c) Calculate the cell potential / Table 24.1. Standard Electrode Potentials 2 Reduction half-reactions Standard reduction potentials Cl2(g)+2e2Cl(aq)Hg2+(aq)+2eHg(l)Ag+(aq)+eAg(s)Fe3+(aq)+eFe2+(aq)O2(g)+2H2O+4e4OH(aq)Cu2+(aq)+2eCu(s)2H3O(aq)+2eH2(g)+2H2ONi2+(aq)+2eNi(s)Cd2+(aq)+2eCd(s)Fe2+(aq)+2eFe(s)Zn2+(aq)+2eZn(s)Al3+(aq)+3eAl(s)Na+(aq)+eNa(s)Li(aq)+eLi(s)1.36V0.85V0.80V0.77V0.40V0.34V0.00V0.28V0.40V0.44V0.76V1.66V2.71V3.05V Oxidation half-reactions Li(s)Li(aq)+eNa(s)Na(aq)+eAl(s)Al3(aq)+3eZn(s)Zn2+(aq)+2eFe(s)Fe2+(aq)+2eCd(s)Cd2+(aq)+2eNi(s)Ni2+(aq)+2eH2(g)+2H2O2H3O(aq)+2eCu(s)Cu2(aq)+2e4OH(aq)O2(g)+2H2O+4eFe(aq)Fe3+(aq)+eAg(s)Ag(aq)+eHg(l)Hg2+(aq)+2e2Cl(aq)Cl2(g)+2e3.05V2.71V1.66V0.76V0.44V0.40V0.28V0.00V0.34V0.40V0.77V0.80V0.85V1.36V 'Standard conditions are: all concentrations =1.00mol/L,T=25.0C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


