Question: PART II. REACTION ORDER (10 POINTS) Consider the reaction below: A + 2B Time, s 0.00 5.00 10.00 15.00 20.00 25.00 30.00 Concentration of A,
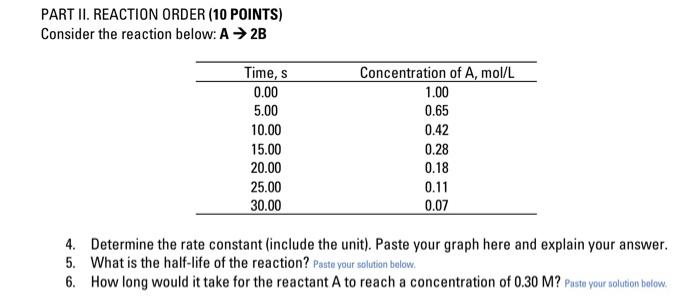
PART II. REACTION ORDER (10 POINTS) Consider the reaction below: A + 2B Time, s 0.00 5.00 10.00 15.00 20.00 25.00 30.00 Concentration of A, mol/L 1.00 0.65 0.42 0.28 0.18 0.11 0.07 4. Determine the rate constant (include the unit). Paste your graph here and explain your answer. 5. What is the half-life of the reaction? Paste your solution below. 6. How long would it take for the reactant A to reach a concentration of 0.30 M? Pusta your solution below
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


