Question: PART III: Sample Problems for solution stoichiometry 1). When 30.0 mL of 0.500 M AgNO3 is added to 25.0 mL of 0.300 M NH4Cl, what
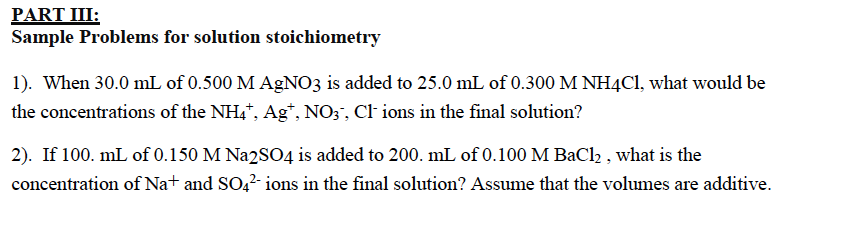
PART III: Sample Problems for solution stoichiometry 1). When 30.0 mL of 0.500 M AgNO3 is added to 25.0 mL of 0.300 M NH4Cl, what would be the concentrations of the NH4+, Ag+, NO3-, Cl- ions in the final solution? 2). If 100. mL of 0.150 M Na2SO4 is added to 200. mL of 0.100 M BaCl2 , what is the concentration of Na+ and SO42- ions in the final solution? Assume that the volumes are additive
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


