Question: PART: The scenes below show two experiments at a given temperature and volume involving reactants X (black) and Y (gree): If the rate law for
PART: The scenes below show two experiments at a given temperature and volume involving reactants X (black) and Y (gree): 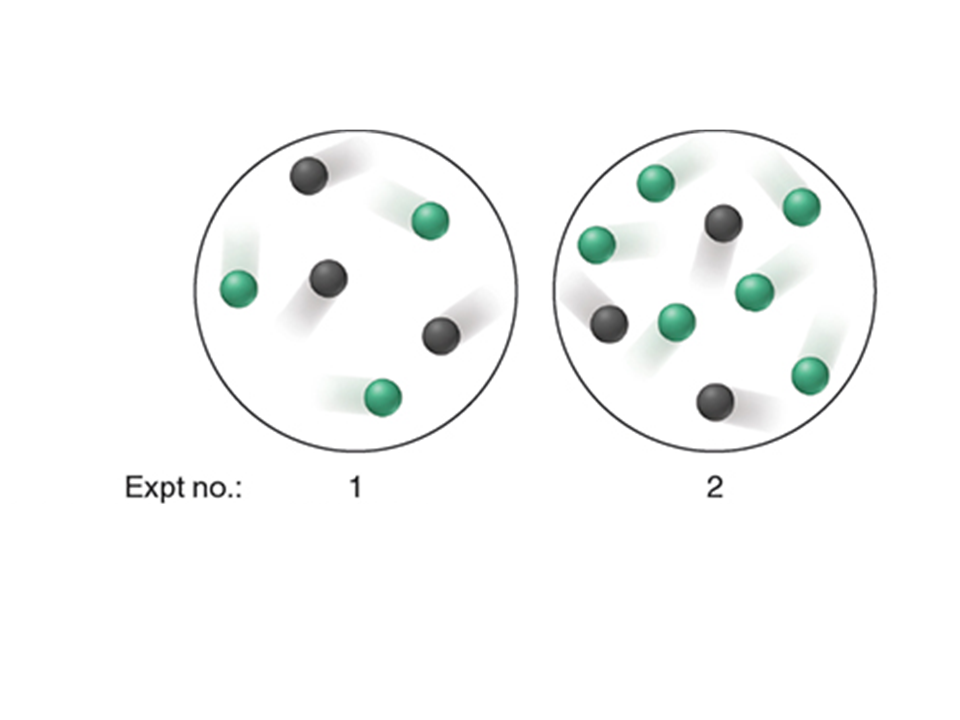
If the rate law for the reaction is rate = k[X]2 what is the initial rate of experiment 2 given that the rate of experiment 1 is 0.50 M/s Part 2:
At a particular temperature and volume, two gases, A (red) and B (blue), react. The following molecular scenes represent starting mixtures for four experiments:
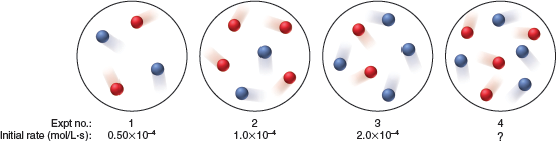
Reaction order with respect to A?
Reaction order with respect to B?
Overall order of the reaction
What is value of k? enter a number to 2 decimal places e.g. 1.23e-4
Part 3:
Find the rate law, the individual and overall reaction orders, and the average value of k for the reaction
H2 + I2 ? 2HI, using the following data at 450?C:
| Exp | initial rate (M/s) | initial H2 (M) | initial I2 (M) |
| 1 | 1.9x10-23 | 0.0113 | 0.0011 |
| 2 | 1.1x10-22 | 0.0220 | 0.0033 |
| 3 | 9.3x10-23 | 0.0550 | 0.0011 |
| 4 | 1.9x10-22 | 0.0220 | 0.0056 |
Order of H2 (enter a number)
Order of I2 (enter a number)
Overall order of the reaction (enter a number)
Value of k (enter a number e.g. 2.3e-20)
Expt no.: 1 2 Expt no.: Initial rate (mol/L-s): 1 0.50x104 2 1.0x10+ 3 2.0x10+
Step by Step Solution
3.36 Rating (165 Votes )
There are 3 Steps involved in it
Lets solve the problems one by one Part 1 Given Rate law textrate kX2 Rate of experiment 1 050 Ms Fo... View full answer

Get step-by-step solutions from verified subject matter experts


