Question: P(atm) PA Main Content Isothermal process PB DB C V (liters) VA VB A 1.00-mol sample of an ideal monatomic gas is taken through the
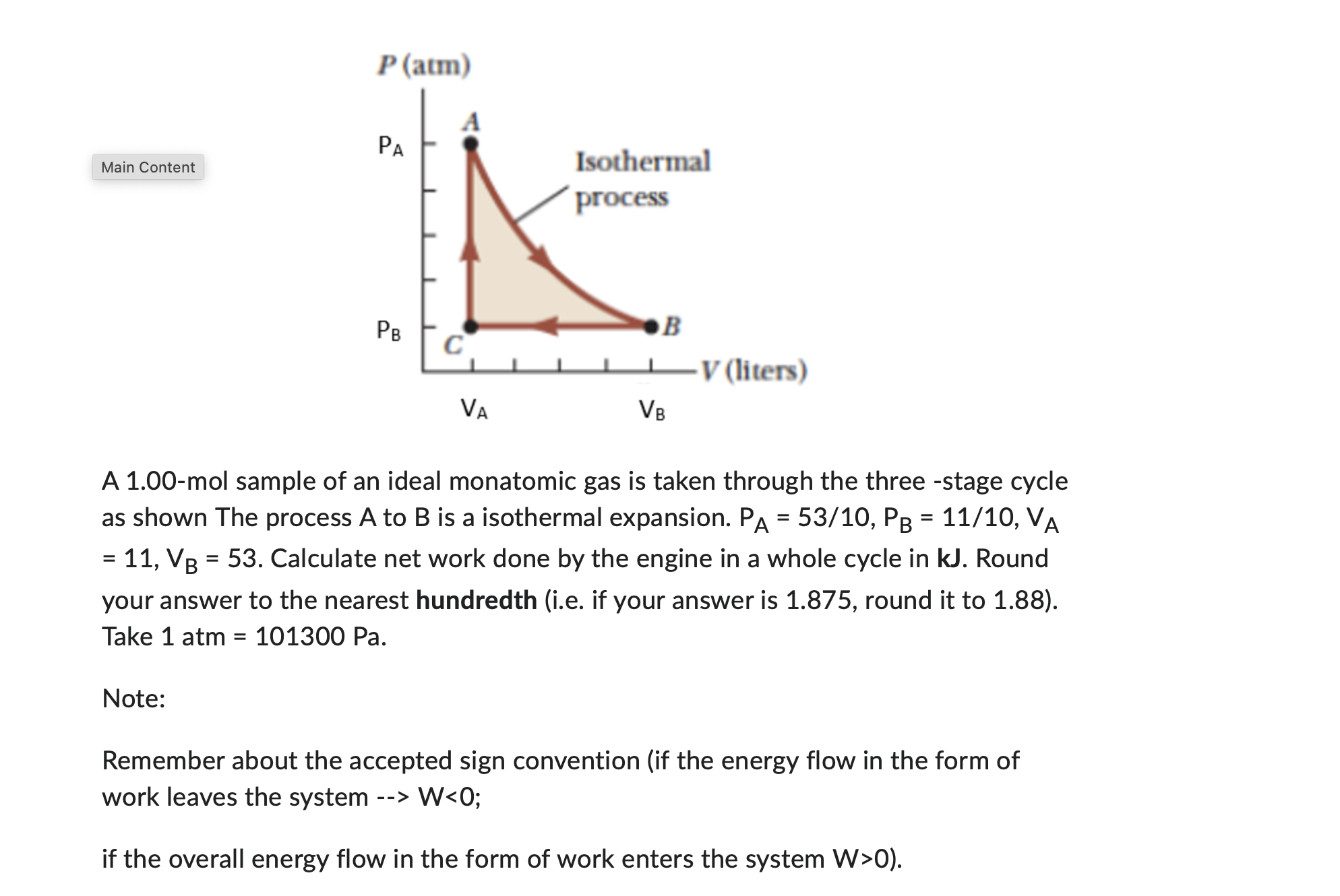
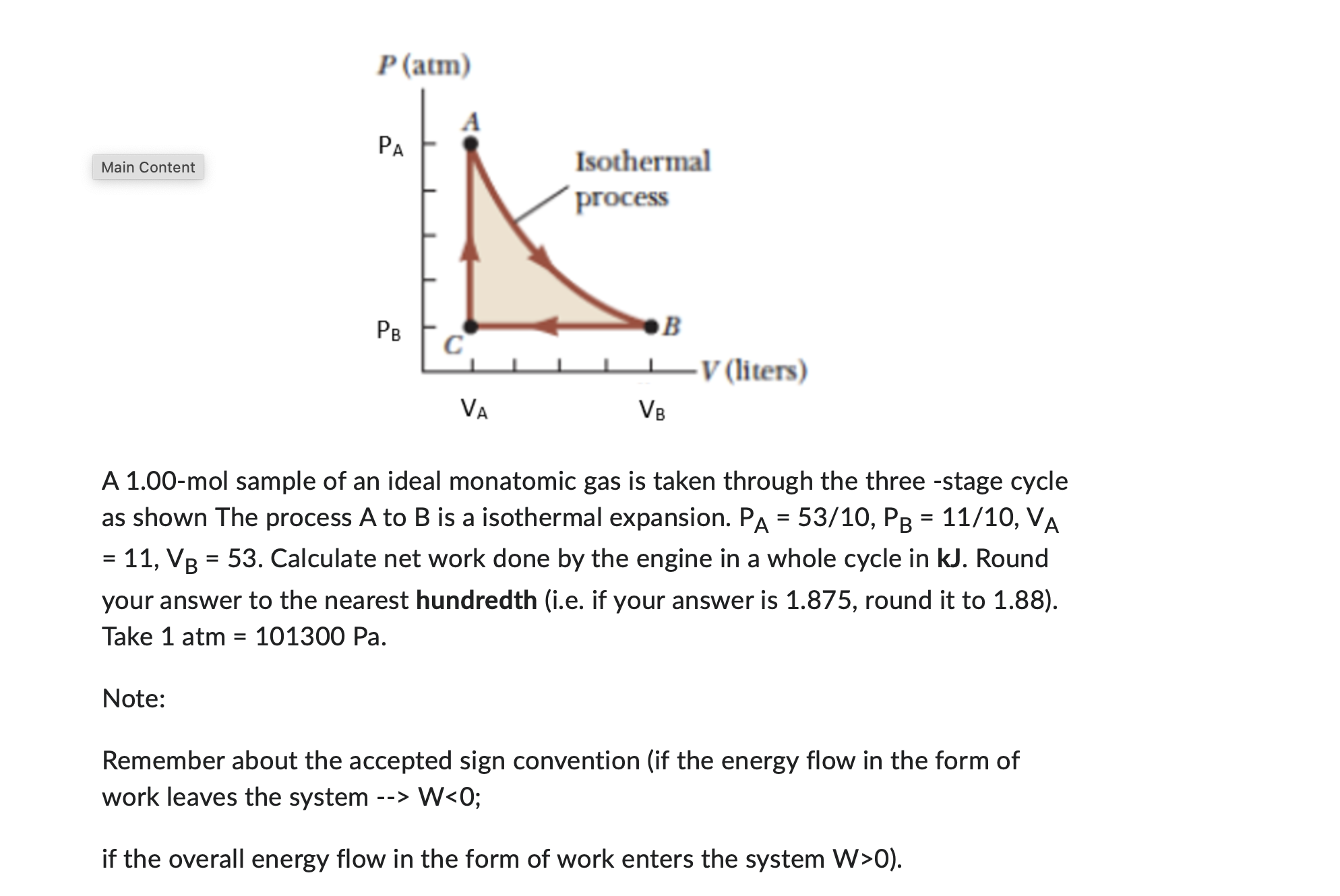
P(atm) PA Main Content Isothermal process PB DB C V (liters) VA VB A 1.00-mol sample of an ideal monatomic gas is taken through the three -stage cycle as shown The process A to B is a isothermal expansion. PA = 53/10, PB = 11/10, VA = 11, VB = 53. Calculate net work done by the engine in a whole cycle in kJ. Round your answer to the nearest hundredth (i.e. if your answer is 1.875, round it to 1.88). Take 1 atm = 101300 Pa. Note: Remember about the accepted sign convention (if the energy flow in the form of work leaves the system --> W
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


