Question: What is the hybridization at the indicated atom in the molecules and ions listed below? Use a wedge-dash drawing, if appropriate, and show all
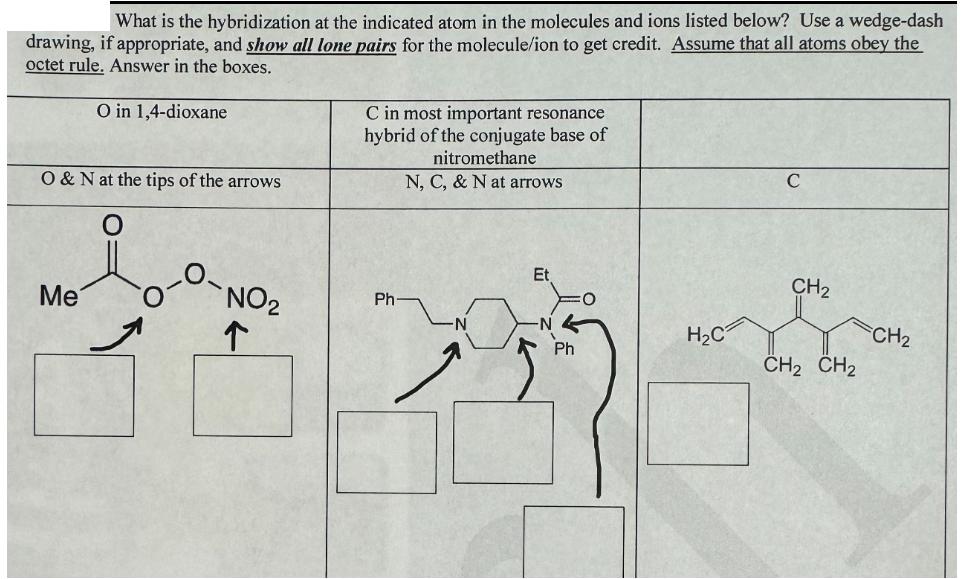
What is the hybridization at the indicated atom in the molecules and ions listed below? Use a wedge-dash drawing, if appropriate, and show all lone pairs for the molecule/ion to get credit. Assume that all atoms obey the octet rule. Answer in the boxes. O in 1,4-dioxane O & N at the tips of the arrows C in most important resonance hybrid of the conjugate base of nitromethane N, C, & N at arrows Me NO2 Ph Et CH2 HC CH2 Ph CH2 CH2
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it
General guidelines Follow the steps kindly O in 14dioxane part has been solved belo... View full answer

Get step-by-step solutions from verified subject matter experts


