Question: Periodic Table For the two compounds given below, one compound obeys the rules of covalent bonding fulfilling the octet rule. The other compound does not
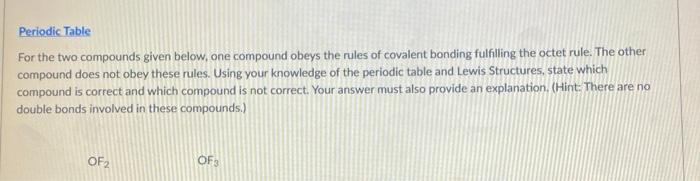
Periodic Table For the two compounds given below, one compound obeys the rules of covalent bonding fulfilling the octet rule. The other compound does not obey these rules. Using your knowledge of the periodic table and Lewis Structures, state which compound is correct and which compound is not correct. Your answer must also provide an explanation. (Hint: There are no double bonds involved in these compounds.) OF 2 OF
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


