Question: Phosphate Buffered Saline (PBS) is the most commonly used buffer in tissue culture. PBS is an isotonic buffer with physiological pH (pH = 7.4) and
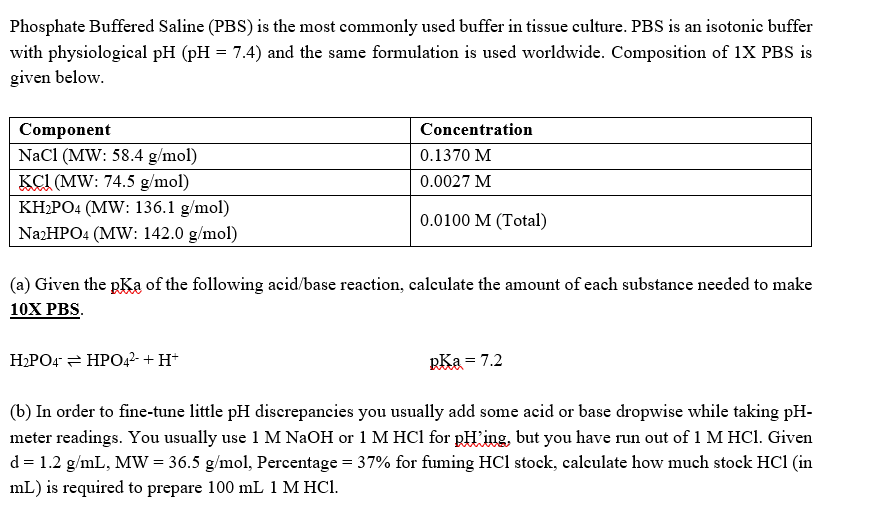
Phosphate Buffered Saline (PBS) is the most commonly used buffer in tissue culture. PBS is an isotonic buffer with physiological pH (pH = 7.4) and the same formulation is used worldwide. Composition of 1X PBS is given below. Component NaCl (MW: 58.4 g/mol) KCI (MW: 74.5 g/mol) KH2PO4 (MW: 136.1 g/mol) Na2HPO4 (MW: 142.0 g/mol) Concentration 0.1370 M 0.0027 M 0.0100 M (Total) (a) Given the pKa of the following acid/base reaction, calculate the amount of each substance needed to make 10X PBS. H2PO4 = HPO42- + H+ RKa = 7.2 (b) In order to fine-tune little pH discrepancies you usually add some acid or base dropwise while taking pH- meter readings. You usually use 1 M NaOH or 1 M HCl for pHing, but you have run out of 1 M HCl. Given d=1.2 g/mL, MW = 36.5 g/mol, Percentage = 37% for fuming HCl stock, calculate how much stock HCl (in mL) is required to prepare 100 mL 1 M HCI
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


