Question: Physical constants The constants for H2O are shown here: - Specific heat of ice: sice=2.09J/(gC) - Specific heat of liquid water: swater=4.18J/(gC) - Enthalpy of
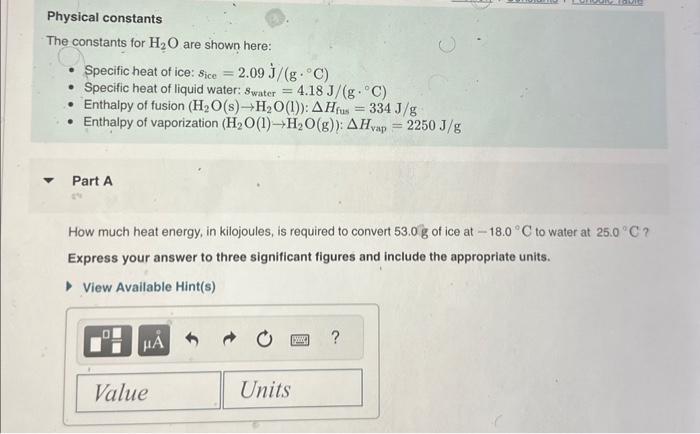
Physical constants The constants for H2O are shown here: - Specific heat of ice: sice=2.09J/(gC) - Specific heat of liquid water: swater=4.18J/(gC) - Enthalpy of fusion (H2O(s)H2O(l)):Hfus=334J/g - Enthalpy of vaporization (H2O(l)H2O(g)):Hvap=2250J/g Part A How much heat energy, in kilojoules, is required to convert 53.0g of ice at 18.0C to water at 25.0C ? Express your answer to three significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


