Question: pleas i need your help with this q this subject is thermodynamics please correct answers immediately you may need this value in selution Q3 Binary
pleas i need your help with this q
this subject is thermodynamics
please correct answers immediately
 you may need this value in selution
you may need this value in selution
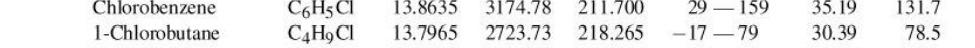
Q3 Binary system of 1-chlorobutane (1) / chorobenzene (2) conforms closely to Raoult's Law. Vapor pressure are given by the following Antoine equations: B: In P, S'' = A T +C; = Where Pis in kPa and T is in C. (a) By constructing a P-X1-y curves at a temperature of 363.15 K, find the bubble point pressure of this mixture (in unit kPa) and the vapor compositions if the liquid phase mole fraction for chorobenzene is 0.4. (b) Find the dew point pressure of this mixture (in unit kPa) and the liquid compositions if the vapor phase mole fraction for chorobenzene is 0.45. - Chlorobenzene 1-Chlorobutane C6H3CI CAH, CI 13.8635 13.7965 3174.78 2723.73 211.700 218.265 - 159 -17 79 35.19 30.39 131.7 78.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


