Question: please answer 2,3,4 using this table 1. Write the reaction for which you are determining the rate. In terms of which reactant or product is

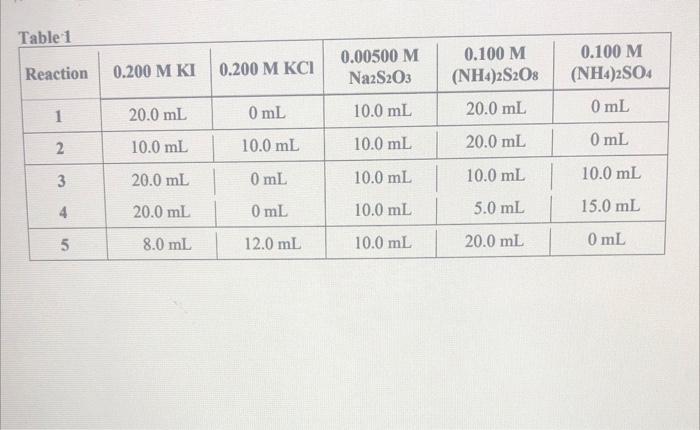
1. Write the reaction for which you are determining the rate. In terms of which reactant or product is the rate being measured in this experiment? For questions 2, 3 and 4, refer to "Reaction I" in Table 1. Show your work. 2. What are the molar concentrations of the reactants in the reaction flask before any reaction takes place? 3. How many moles of iodine, 1, have been produced by the main reaction when the solution turns blue? 4. What is the rate of reaction if the solution turns blue in 30.0 seconds? Table 1 Reaction 0.200 M KI 0.200 M KCI 0.00500 M Na2S203 0.100 M (NH4)2SO4 0.100 M (NH4)2S208 1 20.0 mL 0 mL 10.0 mL 0 mL 20.0 mL 2 10.0 mL 10.0 mL 10.0 mL 20.0 mL 0 mL 3 20.0 mL 0 mL 10.0 mL 10.0 mL 10.0 mL 4 20.0 mL 0 mL 10.0 mL 5.0 mL 15.0 mL 5 8.0 mL 12.0 mL 10.0 mL 20.0 mL 0 mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


