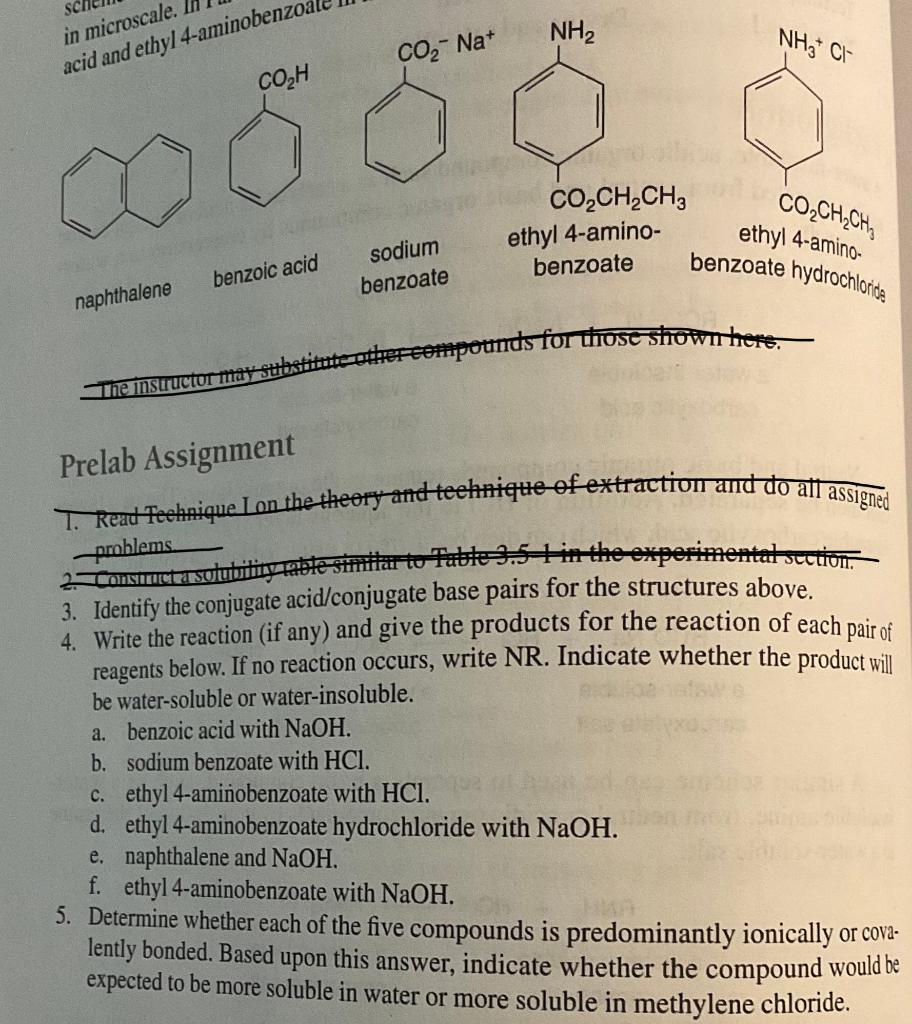
Question: please answer 3,4, and 5 Prelab Assignment 3. Identify the conjugate acid/conjugate base pairs for the structures above. 4. Write the reaction (if any) and
 please answer 3,4, and 5
please answer 3,4, and 5
Prelab Assignment 3. Identify the conjugate acid/conjugate base pairs for the structures above. 4. Write the reaction (if any) and give the products for the reaction of each pair of reagents below. If no reaction occurs, write NR. Indicate whether the product will be water-soluble or water-insoluble. a. benzoic acid with NaOH. b. sodium benzoate with HCl. c. ethyl 4 -aminiobenzoate with HCl. d. ethyl 4-aminobenzoate hydrochloride with NaOH. e. naphthalene and NaOH. f. ethyl 4-aminobenzoate with NaOH. 5. Determine whether each of the five compounds is predominantly ionically or covalently bonded. Based upon this answer, indicate whether the compound would be expected to be more soluble in water or more soluble in methylene chloride
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


