Question: ACID BASE CASE STUDY!! Questions 3, 4, and 5 are a series of acid base questions all related to the reaction below. Hydrolysis of amides
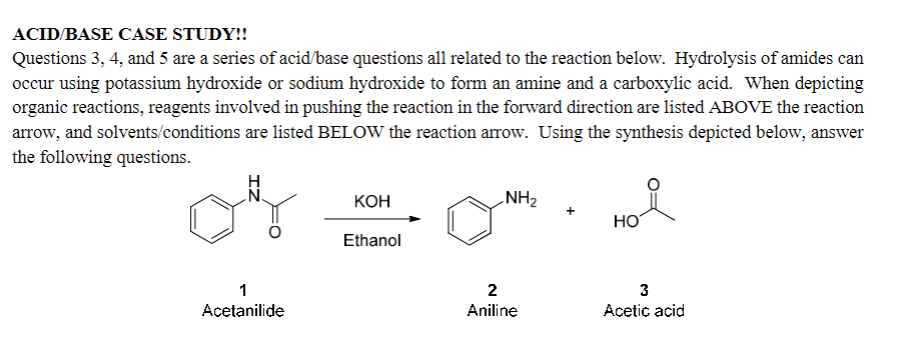
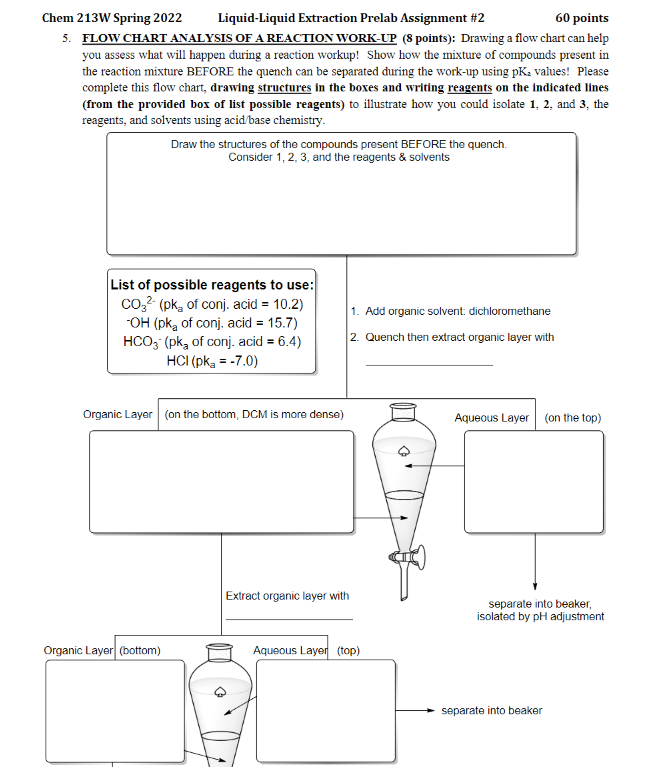
ACID BASE CASE STUDY!! Questions 3, 4, and 5 are a series of acid base questions all related to the reaction below. Hydrolysis of amides can occur using potassium hydroxide or sodium hydroxide to form an amine and a carboxylic acid. When depicting organic reactions, reagents involved in pushing the reaction in the forward direction are listed ABOVE the reaction arrow, and solvents/conditions are listed BELOW the reaction arrow. Using the synthesis depicted below, answer the following questions. KOH NH2 Ethanol 1 Acetanilide 2 Aniline 3 Acetic acid Chem 213W Spring 2022 Liquid-Liquid Extraction Prelab Assignment #2 60 points 5. FLOW CHART ANALYSIS OF A REACTION WORK-UP (8 points): Drawing a flow chart can help you assess what will happen during a reaction workup! Show how the mixture of compounds present in the reaction mixture BEFORE the quench can be separated during the work-up using pK, values! Please complete this flow chart, drawing structures in the boxes and writing reagents on the indicated lines (from the provided box of list possible reagents) to illustrate how you could isolate 1. 2. and 3, the reagents, and solvents using acid base chemistry. Draw the structures of the compounds present BEFORE the quench. Consider 1, 2, 3, and the reagents & solvents List of possible reagents to use: CO2 (pk, of conj. acid = 10.2) OH (pkg of conj, acid = 15.7) HCO3 (pk, of conj. acid = 6.4) HCI (pka = -7.0) 1. Add organic solvent dichloromethane 2. Quench then extract organic layer with Organic Layer on the bottom, DCM is more dense) Aqueous Layer on the top) Extract organic layer with separate into beaker, isolated by pH adjustment Organic Layer (bottom) Aqueous Layer (top) separate into beaker
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


