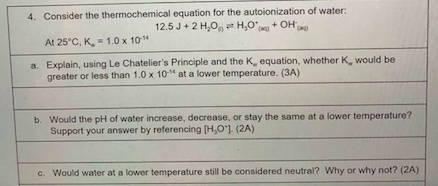
Question: please answer 4 a b c 4. Consider the thermochemical equation for the autoionization of water: 12.5 J + 2H,0,H0+ OH At 25C, K, -
please answer 4 a b c
4. Consider the thermochemical equation for the autoionization of water: 12.5 J + 2H,0,H0+ OH At 25C, K, - 1.0 x 10-14 a. Explain, using Le Chatelier's Principle and the K equation, whether K, would be greater or less than 1.0 x 10" at a lower temperature. (3A) b. Would the pH of water increase, decrease, or stay the same at a lower temperature? Support your answer by referencing [H,O") (2A) c. Would water at a lower temperature still be considered neutral? Why or why not? (2A)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


