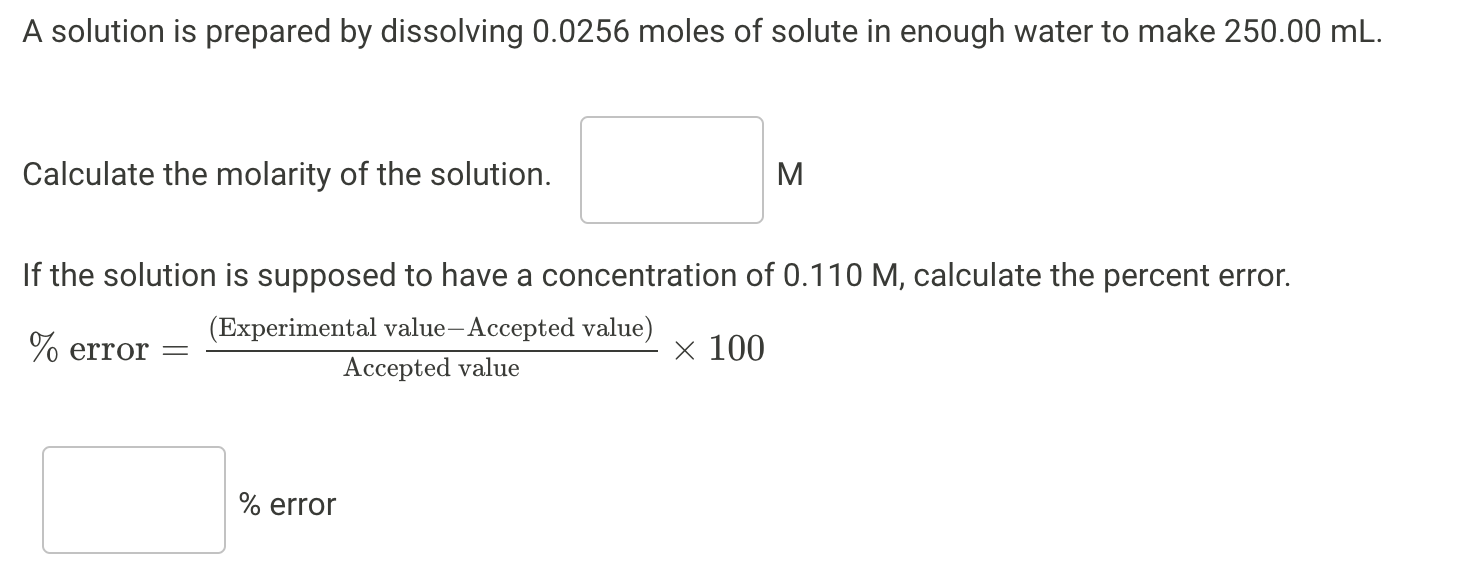
Question: please answer 6a and 6b A solution is prepared by dissolving 0.0256 moles of solute in enough water to make 250.00 mL. Calculate the molarity

please answer 6a and 6b
A solution is prepared by dissolving 0.0256 moles of solute in enough water to make 250.00 mL. Calculate the molarity of the solution. M a If the solution is supposed to have a concentration of 0.110 M, calculate the percent error. % error = (Experimental value-Accepted value) x 100 Accepted value % error Question 10 Status: Not yet answered | Points possible: 2.00 Prepare for lab by opening the Experiment Worksheet and working through the calculations in advance. Scenario A: 100.00 mL of a 0.200 M carbonate ion solution You have eagerly volunteered to aid in your professor's chemical research endeavor. Since you are fairly new in the game, you have been provided with the task of preparing 100.00 mL of a 0.200 M carbonate ion solution. After preparing the solution, you should bring it to your professor for a conductivity test. The results of this measurement will indicate the accuracy of your solution preparation by comparing the conductivity of your solution to a standard value. Look over the glassware and reagents provided to you, formulate a strategy, and complete the task at hand! How many moles of carbonate ion are present in 100.00 mL of a 0.200 M carbonate ion solution? Report your answer with correct significant figures and show your work on the Experiment Worksheet. moles of carbonate What mass of potassium carbonate contains the necessary number of moles of carbonate ion? Report your answer with correct significant figures and show your work on the Experiment Worksheet. g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


