Question: please answer all 3 problems correctly for a thumbs up Each ionizable group of an amino acid can exist in one of two states, charged
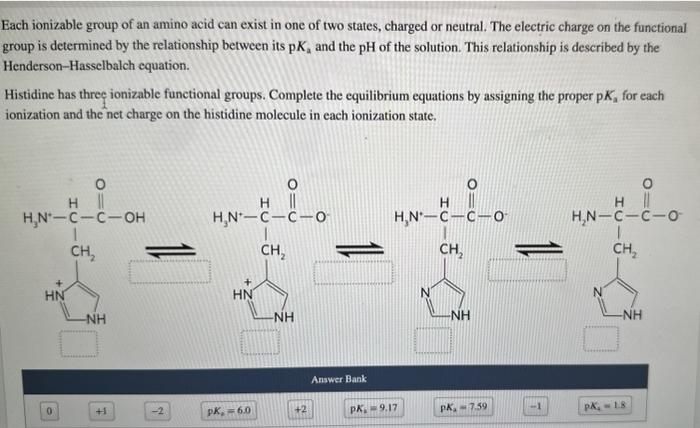
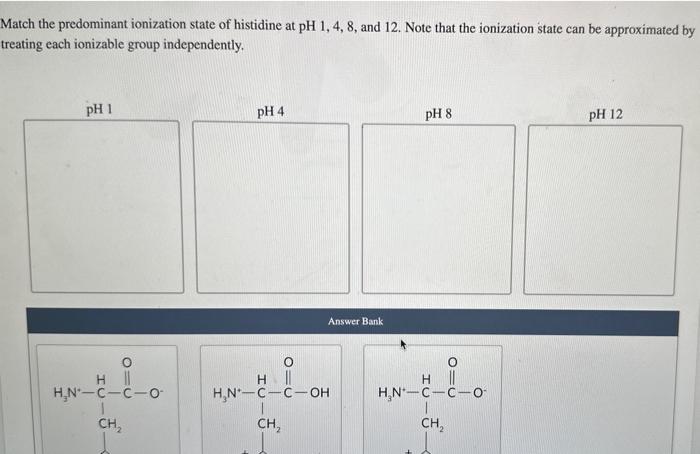
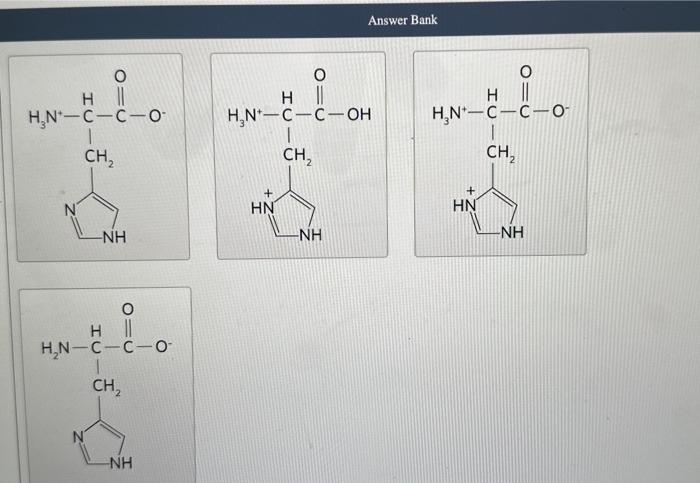
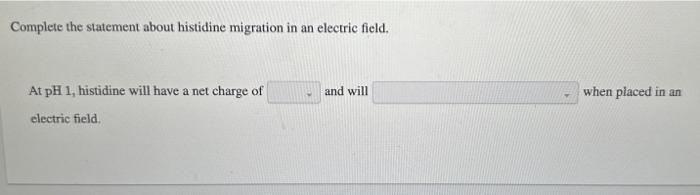
Each ionizable group of an amino acid can exist in one of two states, charged or neutral. The electric charge on the functional group is determined by the relationship between its pKa and the pH of the solution. This relationship is described by the Henderson-Hasselbalch equation. Histidine has three ionizable functional groups. Complete the equilibrium equations by assigning the proper p Ka for each ionization and the net charge on the histidine molecule in each ionization state. Match the predominant ionization state of histidine at pH1,4,8, and 12 . Note that the ionization state can be approximated by reating each ionizable group independently. Answer Bank Complete the statement about histidine migration in an electric field. At pH 1 , histidine will have a net charge of and will when placed in an electric field. Each ionizable group of an amino acid can exist in one of two states, charged or neutral. The electric charge on the functional group is determined by the relationship between its pKa and the pH of the solution. This relationship is described by the Henderson-Hasselbalch equation. Histidine has three ionizable functional groups. Complete the equilibrium equations by assigning the proper p Ka for each ionization and the net charge on the histidine molecule in each ionization state. Match the predominant ionization state of histidine at pH1,4,8, and 12 . Note that the ionization state can be approximated by reating each ionizable group independently. Answer Bank Complete the statement about histidine migration in an electric field. At pH 1 , histidine will have a net charge of and will when placed in an electric field
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


