Question: please answer all 4 :) 2. A helium balloon has a volume ot 2.30 int 23.5C and a pressure of 1.00 atm at sea level.

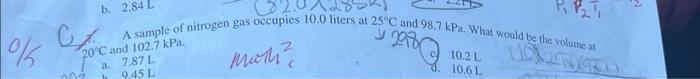
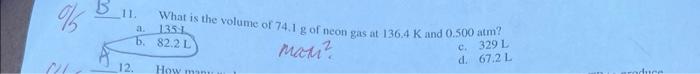

2. A helium balloon has a volume ot 2.30 int 23.5C and a pressure of 1.00 atm at sea level. The balloon is released and floats upward. At a certain height the atmospheric pressure is 0.810 atm and the temperature is 12.0C. Calculate the new volume of the balloon. a. 2.73L b. 2.84L 1. A sample of nitrogen gas occupies 10.0 liters at 25C and 98.7kPa, What would be the volunie at a. 7.87L. nictic d) 10.2L d. 10.6L 5 11. What is the volume of 74.1g of neon gas at 136.4K and 0.500atm ? a. 1351 mant d. 67.2L 12. How many moles of CH4(g) are needed to react completely with 520L of O2(g) at STP to produce CO2(g) and H2O(g) ? (You will need to write a balanced equation.) . 11.6min 10014= c=4.64molC=120 dh 2.32mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


