Question: please answer all for UPVOTE P-Chemistry 2 Select which statement best describes the effect on the diagram if the temperature was decreased.. in 10 +
please answer all for UPVOTE
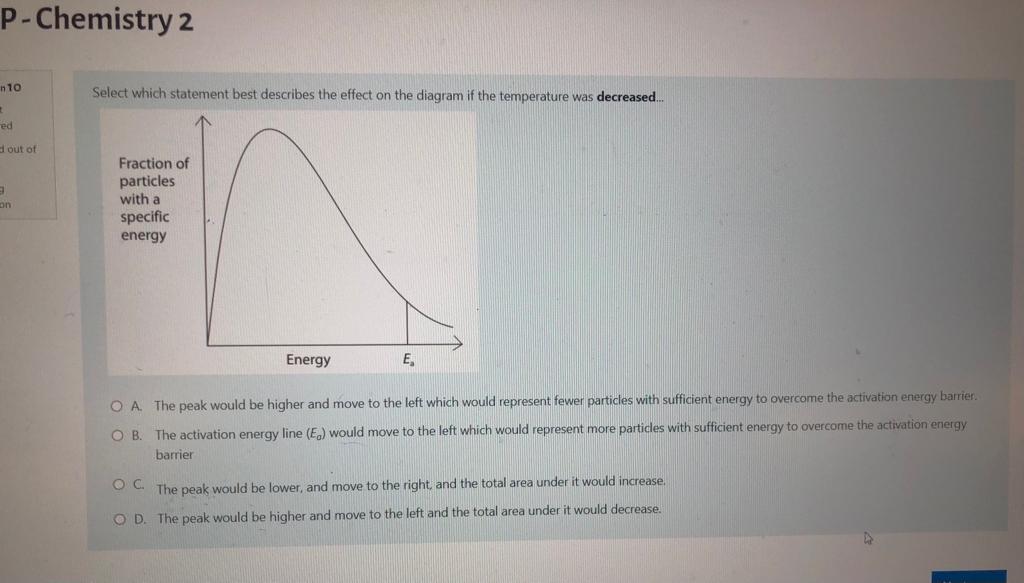
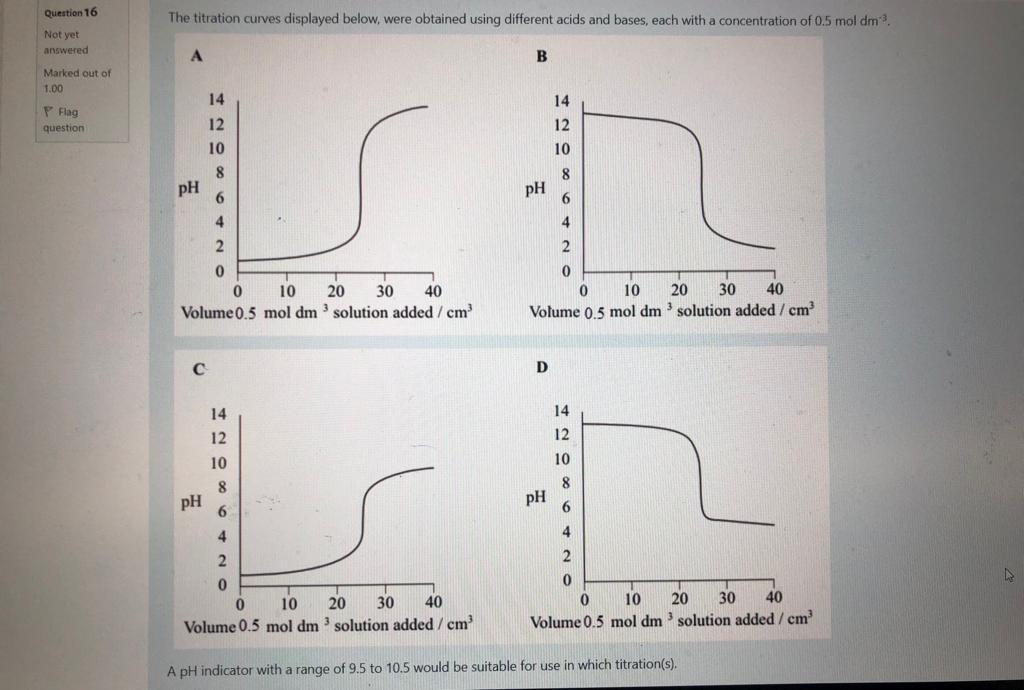
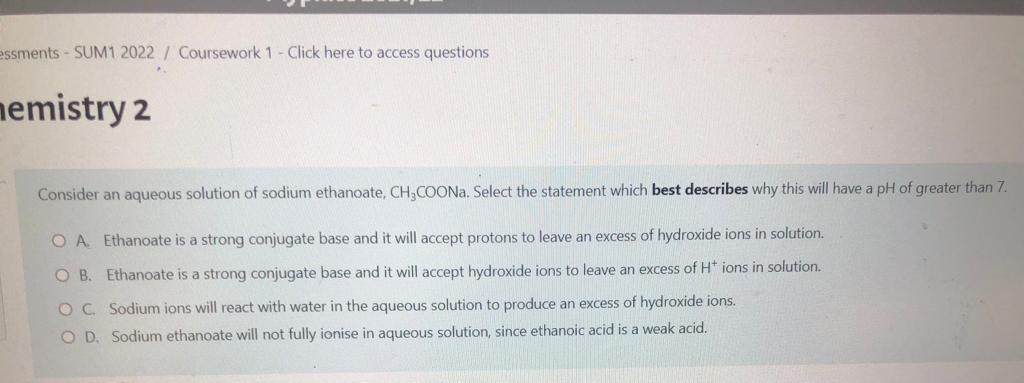

P-Chemistry 2 Select which statement best describes the effect on the diagram if the temperature was decreased.. in 10 + ed out of a Fraction of particles with a specific energy Energy E, O A. The peak would be higher and move to the left which would represent fewer particles with sufficient energy to overcome the activation energy barrier. O B. The activation energy line (E) would move to the left which would represent more particles with sufficient energy to overcome the activation energy barrier OC. The peak would be lower, and move to the right and the total area under it would increase. OD. The peak would be higher and move to the left and the total area under it would decrease. Question 16 Not yet answered Marked out of 1.00 Flag question The titration curves displayed below, were obtained using different acids and bases, each with a concentration of 0.5 mol dm. A B 8 8 6 6 4 4 2 2 0 0 0 10 20 30 40 Volume 0.5 mol dm 3 solution added / cm 0 10 20 30 40 Volume 0.5 mol dm 3 solution added / cm C D 8 8 6 6 4 4 2 2 0 0 U 10 0 30 40 0 20 Volume 0.5 mol dm 3 solution added / cm 40 20 30 10 Volume 0.5 mol dm3 solution added / cm A pH indicator with a range of 9.5 to 10.5 would be suitable for use in which titration(s). PH PH 14 12 10 14 12 10 PH pH 14 12 10 14 12 10 essments - SUM1 2022 / Coursework 1 - Click here to access questions nemistry 2 Consider an aqueous solution of sodium ethanoate, CH3COONa. Select the statement which best describes why this will have a pH of greater than 7. OA Ethanoate is a strong conjugate base and it will accept protons to leave an excess of hydroxide ions in solution. OB. Ethanoate is a strong conjugate base and it will accept hydroxide ions to leave an excess of H* ions in solution. OC. Sodium ions will react with water in the aqueous solution to produce an excess of hydroxide ions. O D. Sodium ethanoate will not fully ionise in aqueous solution, since ethanoic acid is a weak acid. Question 12 Not yet answered Marked out of 1.00 Flag question Previous page Consider a reaction with the following rate equation: ratek [A] [B] [C] Select which statement best describes what will happen to the rate of the reaction if [A] is doubled, and [B] is halved. O A. The rate will decrease by a factor of 2. OB. The rate will stay the same. O C. The rate will increase by a factor of 2. OD. The rate will increase by a factor 8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


