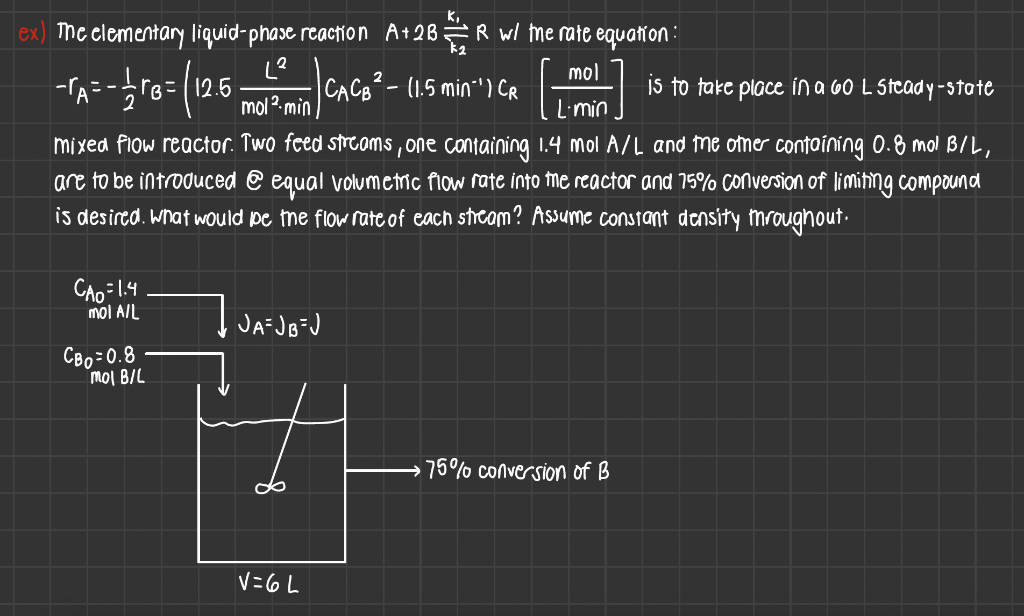
Question: Please write down step by step. Thanks!!! 2 - =-370=(125 more on ) [H 2 Limin ex) The elementary liquid-phase reaction A+2BFR wi me rate
Please write down step by step. Thanks!!!
2 - =-370=(125 more on ) [H 2 Limin ex) The elementary liquid-phase reaction A+2BFR wi me rate equation : L? mol -- 12.6 | CACB - 11.5 min "I CR is to take place in a 60 L Steady-state mola min mixed Fiow reactor. Two feed streams, one containing 1.4 mol A/L and me other containing 0.8 mol BIL, are to be introduced @ equal volumetric flow rate into me reactor and 75% conversion of limining compound is desired. What would be the flow rate of each stream? Assume constant density mroughout. CAo=1.4 MOI AIL I JA=JBU CBp=0.8 Mol B/L 75% conversion of B V=6L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


