Question: please answer all. help asap. a) Calibration certe Prepare a calibration curve ring standard solution Prepare your standards and make sure the spectrometer is set
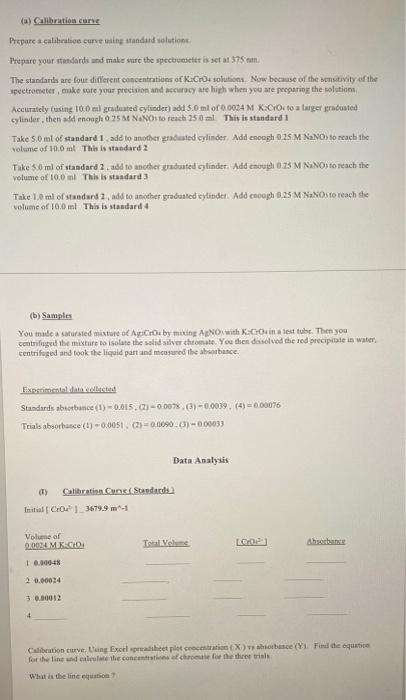
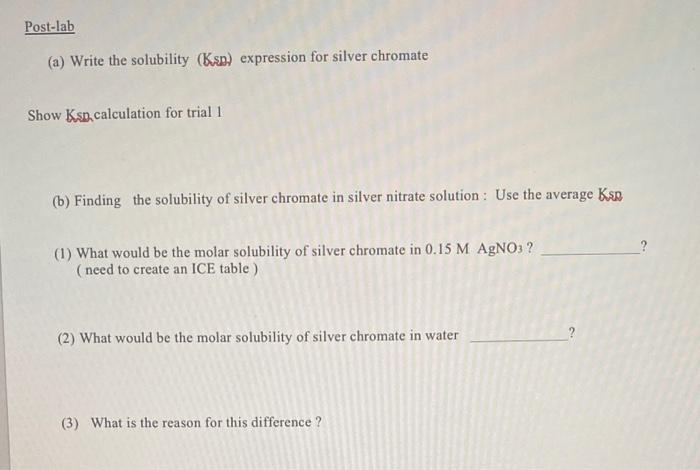
a) Calibration certe Prepare a calibration curve ring standard solution Prepare your standards and make sure the spectrometer is set at 375 cm The standards are four different concentrations of CrO solutions. Now because of the sensitivity of the spectrometer, make sure your precision and cracy are high when you are preparing the solutions Accurately (using 100 ml graduated cylinder) add 5.0 ml of 0.0004 M .Co to a larger graduated cylinder, then mooch 25 N.NO to reach 250ml This is standard 1 Take 5.0 ml of standard 1. add to another graduated cylinder. Add enough 025 M NaNO) to reach the volume of 100 ml This is standard 2 Take 50 ml of standard 2. add to another graduated cylinder. Add enough 0.25 M N.NO) to reach the volume of 10,0 ml This is standard Take 1 ml of standard 1, add to another graduated cylinder. Add enough 025 M N.NO to reach the volume of 100 ml This is standard 4 (b) Samples You made a stunned mixture of ACO by mixing ANO with contest tube. Then you centrifuged the mixture to isolate the solid silver chromate You then dissolved the red precipitate in water, centrifuged and took the liquid part and measured the whance. Experimental detected Standards borbince (1) -0.015.) -0.0078.0) -0.0039 (1) 000076 Trials absorhunce (1) -0.0051 (7) 0.0090.01 -0.00033 Data Analysis (1) Calibration Curs Standards Initial COP) 3679.9 m-1 Volume of 0.0024 MKOA TY LCO Ahsan 1 0.00048 2 0.00024 3 0.00012 Calibration curve tung Excel pradsheet pleteocent X) hobice (Yu Find the equatice for the line und lie the contact for the three trial What is the lineston Post-lab (a) Write the solubility (Ksp) expression for silver chromate Show Ksn calculation for trial 1 (b) Finding the solubility of silver chromate in silver nitrate solution: Use the average Kan ? (1) What would be the molar solubility of silver chromate in 0.15 M AgNO3 ? (need to create an ICE table) (2) What would be the molar solubility of silver chromate in water ? (3) What is the reason for this difference
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


