Question: please answer all i will leave a like thank you 2. How much energy is contained in each of the following? a. 3 moles of

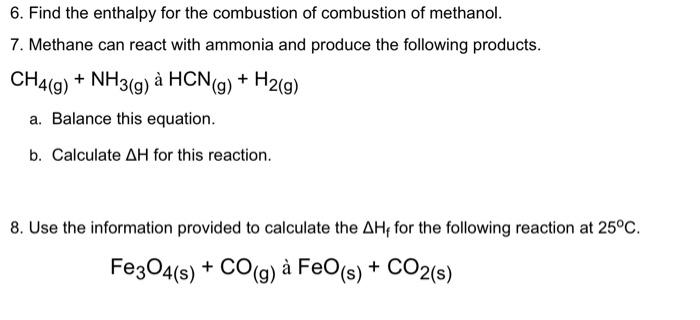
2. How much energy is contained in each of the following? a. 3 moles of iodine molecules b. 5 moles of CO2 molecules c. 4.8 moles of butane 3. Make an energy diagram for the heat formation of one mole of each of the following substances: a. H2+I2 2HI b. 2H2(9)+CO(9)CH3OH() c. N2+4H2 2NH4+ 4. If the change in enthalpy for carbon monoxide (CO) is 104.5kJ/mol, and that of carbon dioxide (CO2) is 392.92kJ/mol, what can we conclude about the stability of these two substances? 5. Calculate the change in enthalpy for the following reactions: a. C2H()+O2()CO2()+H2O(0) b. C25H52(g)+O2(g)CO2(g)+H2O(1) c. N2(g)+H2(g)NH3(g) d. C(s)+1/2O2()CO() 6. Find the enthalpy for the combustion of combustion of methanol. 7. Methane can react with ammonia and produce the following products. CH4(g)+NH3(g)aHCN(g)+H2(g) a. Balance this equation. b. Calculate H for this reaction. 8. Use the information provided to calculate the Hf for the following reaction at 25C. Fe3O4(s)+CO(g)aFeO(s)+CO2(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


