Question: Please answer all of it Problem 3. Kinetics (6 points) When heated, cyclopropane can undergo a unimolecular rearrangement process to form propene in a first-order
Please answer all of it
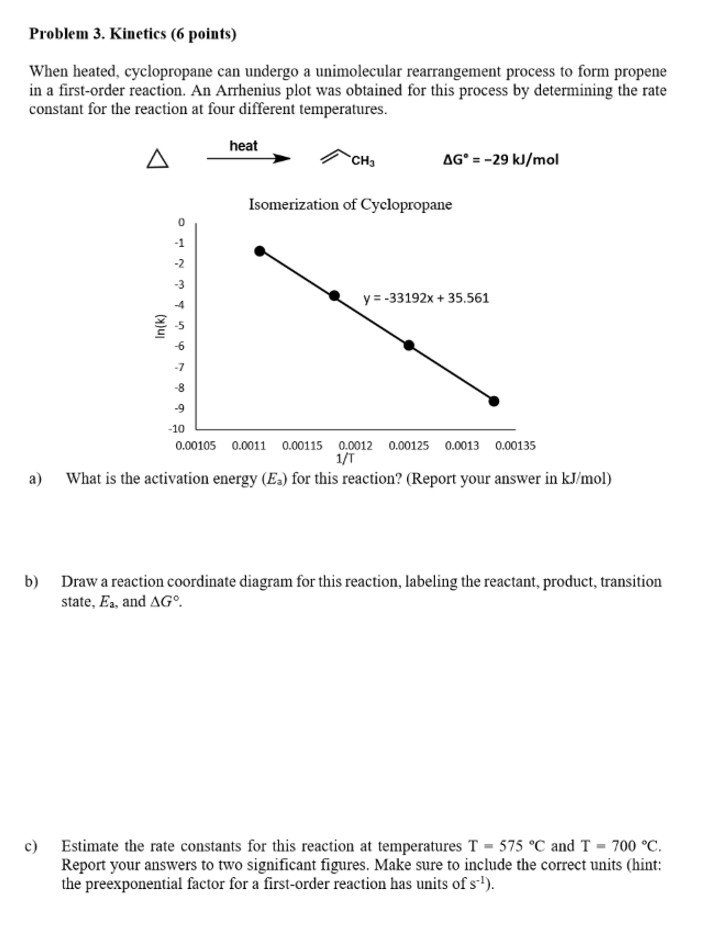
Problem 3. Kinetics (6 points) When heated, cyclopropane can undergo a unimolecular rearrangement process to form propene in a first-order reaction. An Arrhenius plot was obtained for this process by determining the rate constant for the reaction at four different temperatures. heat A . AG = -29 kJ/mol Isomerization of Cyclopropane -1 -2 -3 y=-33192x + 35.561 -4 Ink) -6 -7 -8 -9 -10 0.00105 0.0011 0.00115 0.0012 0.00125 0.0013 0.00135 1/1 What is the activation energy (Es) for this reaction? (Report your answer in kJ/mol) a) b) Draw a reaction coordinate diagram for this reaction, labeling the reactant, product, transition state, Ea, and AG c) Estimate the rate constants for this reaction at temperatures T - 575 C and T - 700 C. Report your answers to two significant figures. Make sure to include the correct units (hint: the preexponential factor for a first-order reaction has units of st)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


