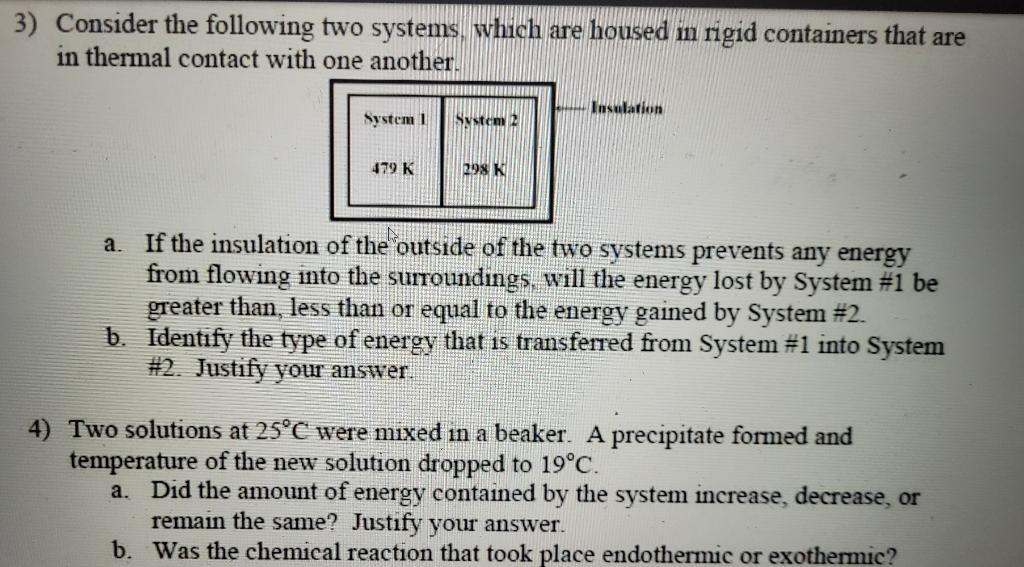
Question: please answer all parts Consider the following two systems, which are housed in rigid containers that are in thermal contact with one another. a. If
 please answer all parts
please answer all parts
Consider the following two systems, which are housed in rigid containers that are in thermal contact with one another. a. If the insulation of the outside of the two systems prevents any energy from flowing into the surroundings, will the energy lost by System \#1 be greater than, less than or equal to the energy gained by System \#2. b. Identify the type of energy that is transferred from System \#1 into System \#2. Justify your answer. 4) Two solutions at 25C were mixed in a beaker. A precipitate formed and temperature of the new solution dropped to 19C. a. Did the amount of energy contained by the system increase, decrease, or remain the same? Justify your answer. b. Was the chemical reaction that took place endothermic or exothermic
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


