Question: PLEASE ANSWER ALL PARTS FOR . Please do not leave incomplete. thank you so much! dont need explanations. Use the following information to answer the
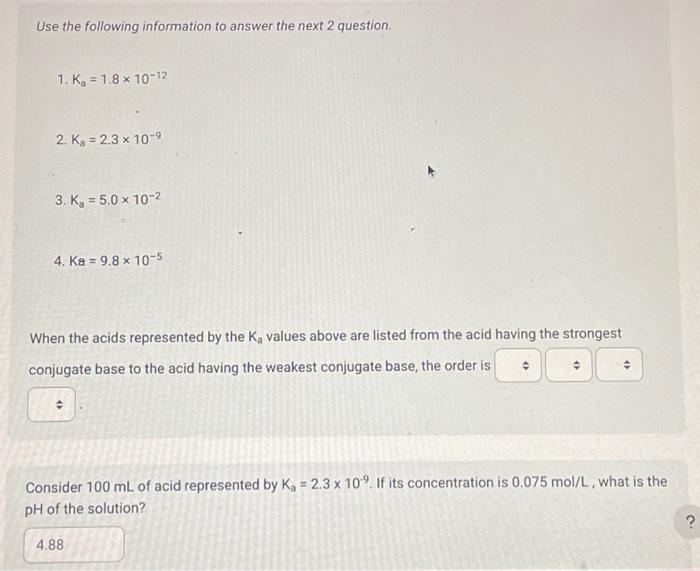
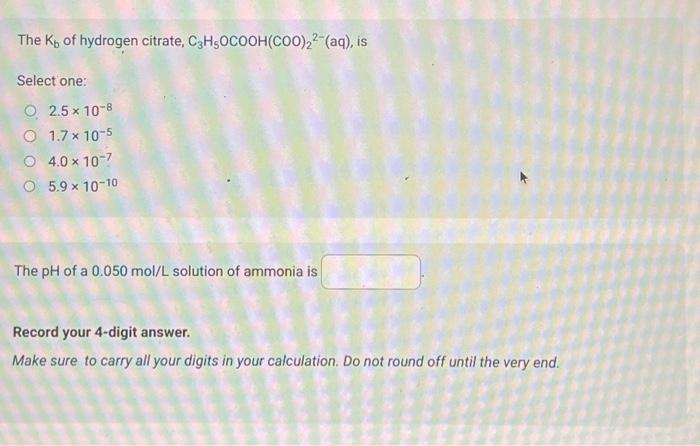

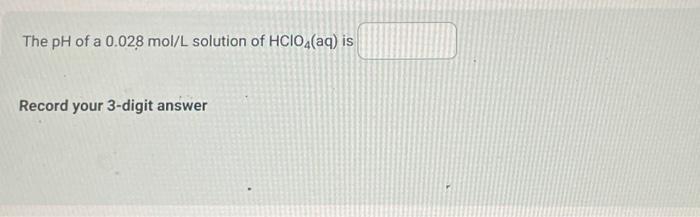

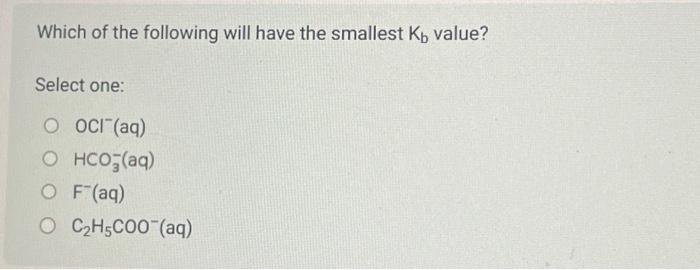
Use the following information to answer the next 2 question. 1. Ka=1.81012 2. K3=2.3109 3. Ka=5.0102 4. Ka=9.8105 When the acids represented by the Ka values above are listed from the acid having the strongest conjugate base to the acid having the weakest conjugate base, the order is Consider 100mL of acid represented by Ka=2.3109. If its concentration is 0.075mol/L, what is the pH of the solution? The Kb of hydrogen citrate, C3H5OCOOH(COO)22(aq), is Select one: 2.51081.71054.01075.91010 The pH of a 0.050mol/L solution of ammonia is Record your 4-digit answer. Make sure to carry all your digits in your calculation. Do not round off until the very end. If a 100mL sample of potassium hypochlorite (KOCl(aq)) has a pH of 9.75 at 25.0C, then the concentration of the sample of potassium hypochlorite expressed in scientific notation is a.b 10c mol/L. The values of a,b, and c are The pH of a 0.028mol/L solution of HClO4(aq) is Record your 3-digit answer The Kb of HCO3(aq) is Select one: 2.1104 4.5107 2.2108 4.71011 Which of the following will have the smallest Kb value? Select one: OCl(aq) HCO3(aq) F(aq) C2H5COO(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


