Question: PLEASE ANSWER ALL PARTS FOR . Please do not leave incomplete. thank you so much! Use the following information to answer the next 4 questions.

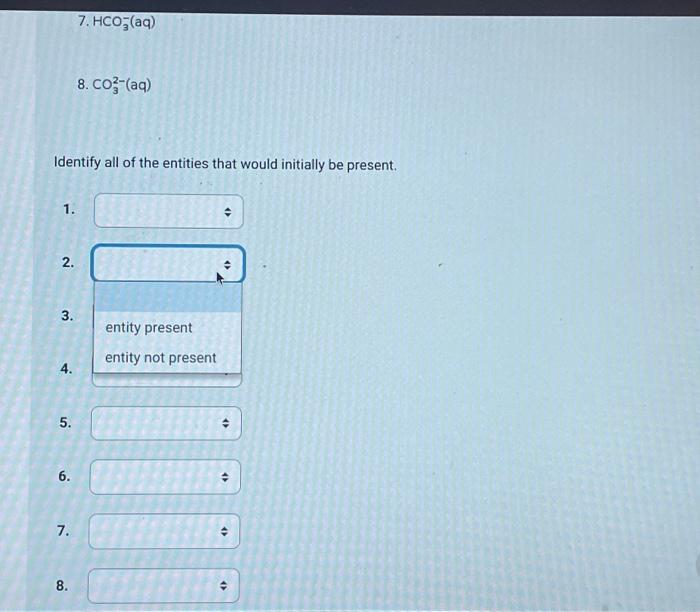
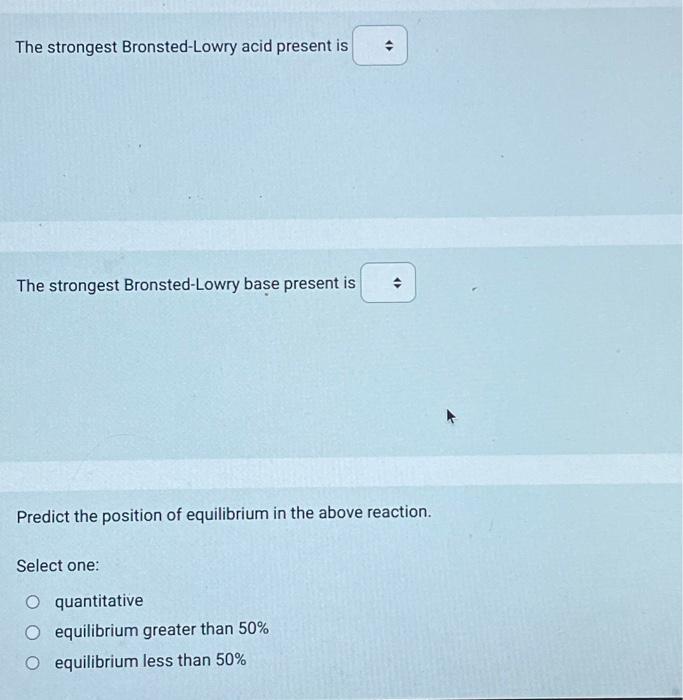
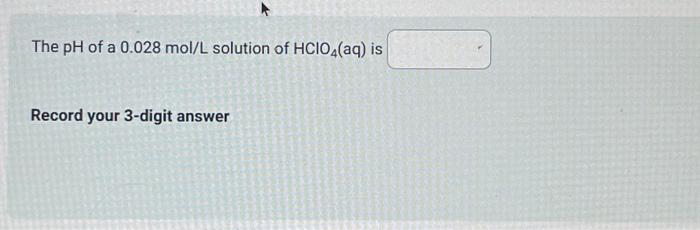
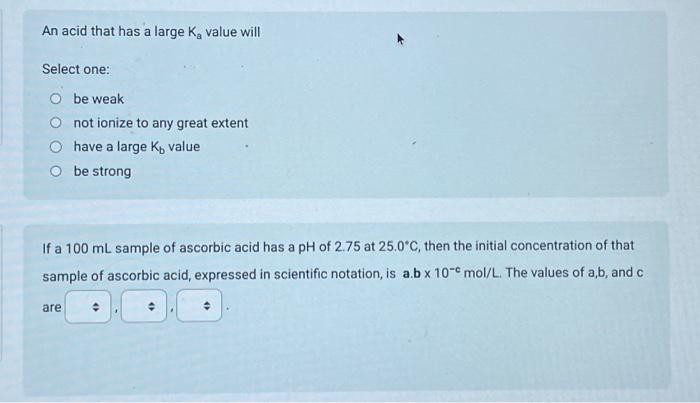
Use the following information to answer the next 4 questions. A technician combined 100mL of 0.10mol/L tellurous acid (H2TeO3(aq)) with 100mL of 0.10mol/L sodium hydrogen carbonate. 1. H2TeO3 (aq) Ka=3.3103 2. H3O+(aq) 3. HTeO3(aq)Ka=2.0108 4. H2O(l) 5. NaHCO3 (aq) 6. Na+(aq) 7. HCO3(aq) Identify all of the entities that would initially be present. The strongest Bronsted-Lowry acid present is The strongest Bronsted-Lowry base present is Predict the position of equilibrium in the above reaction. Select one: quantitative equilibrium greater than 50% equilibrium less than 50% The pH of a 0.028mol/L solution of HClO4(aq) is Record your 3-digit answer An acid that has a large Ka value will Select one: be weak not ionize to any great extent have a large Kb value be strong If a 100mL sample of ascorbic acid has a pH of 2.75 at 25.0C, then the initial concentration of that sample of ascorbic acid, expressed in scientific notation, is a.b10cmol/L. The values of a,b, and c are
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


