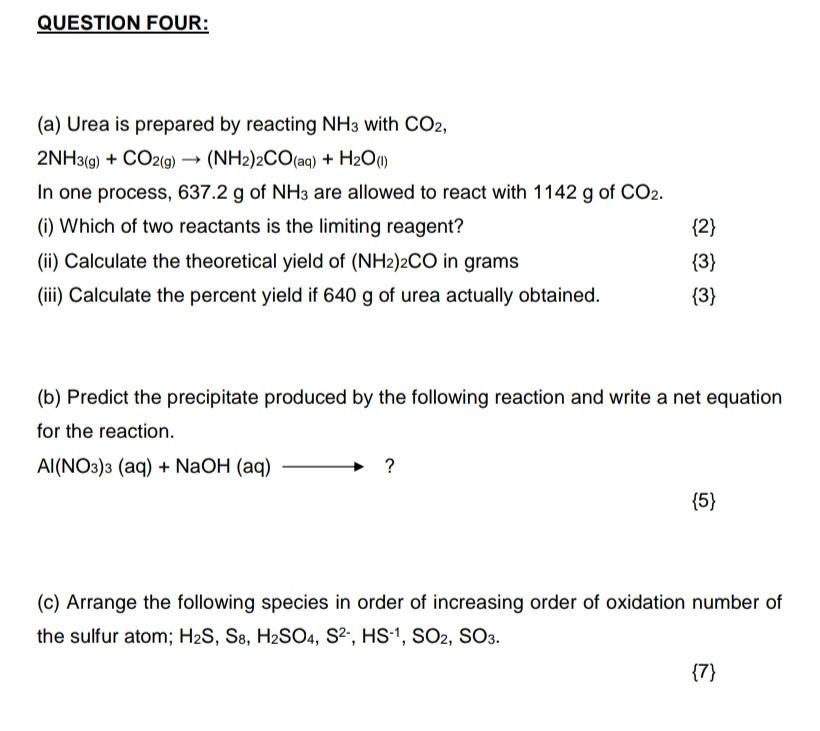
Question: Please answer all questions and I will upvote Answering part questions won't help me Thank you (a) Urea is prepared by reacting NH3 with CO2,
Please answer all questions and I will upvote
Answering part questions won't help me
Thank you
(a) Urea is prepared by reacting NH3 with CO2, 2NH3(g)+CO2(g)(NH2)2CO(aq)+H2O(1) In one process, 637.2g of NH3 are allowed to react with 1142g of CO2. (i) Which of two reactants is the limiting reagent? {2} (ii) Calculate the theoretical yield of (NH2)2CO in grams {3} (iii) Calculate the percent yield if 640g of urea actually obtained. {3} (b) Predict the precipitate produced by the following reaction and write a net equation for the reaction. Al(NO3)3(aq)+NaOH(aq)? {5} (c) Arrange the following species in order of increasing order of oxidation number of the sulfur atom; H2S,S8,H2SO4,S2,HS1,SO2,SO3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


