Question: Please answer all thank you! Last question: Analyze the statement. For a molecule to be polar, the presence of polar bonds is necessary, but it
Please answer all thank you!
Last question: Analyze the statement. For a molecule to be polar, the presence of polar bonds is necessary, but it is not a sufficient requirement.
1. If a molecule does not have one (or more) polar bonds...
a) it can be polar, depending on the hybridization of the molecule
b) it cannot possess a dipole moment and, therefore, it cannot be polar
c) it will be polar, since the polarity of a molecule is not dependent on the polarity of its bonds. All molecules possess a dipole moment.
2. If a molecule has polar bonds...
a) it will be polar, since there will be a dipole moment across the polar bond
b) it may not be polar, if the bonds are directed so that the bond moments cancel
c) it will not be polar, since the bond moments will cancel each other
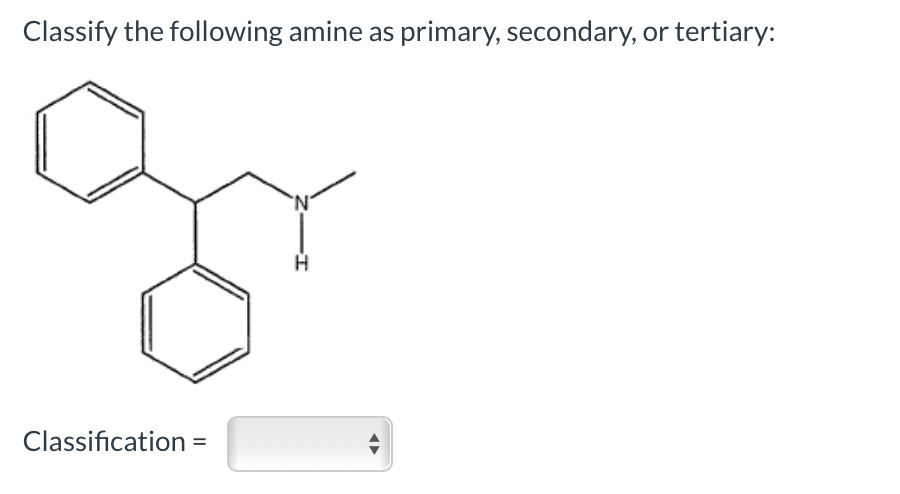
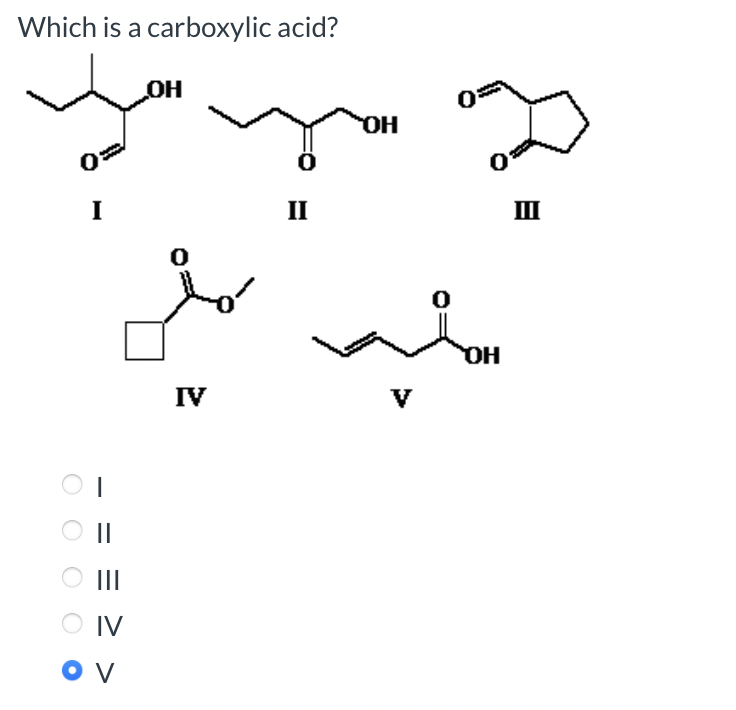
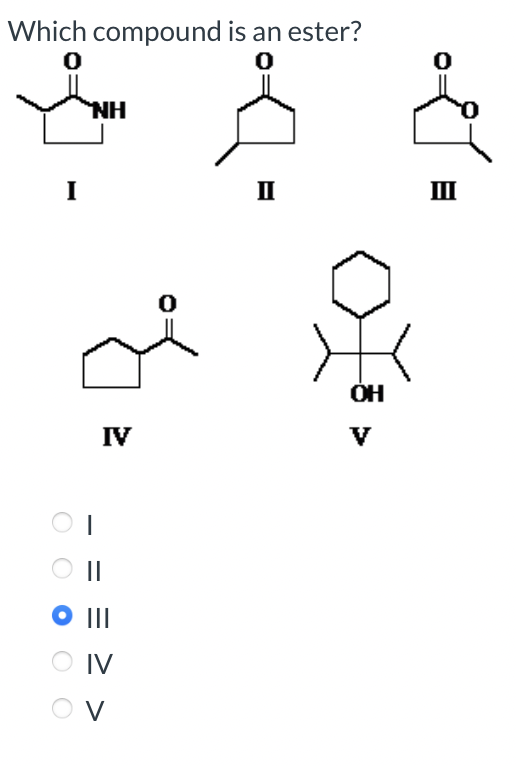
Classify the following amine as primary, secondary, or tertiary: Classification = Which is a carboxylic acid? I II IV I II III Which compound is an ester? I II IV V I II III IV
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


