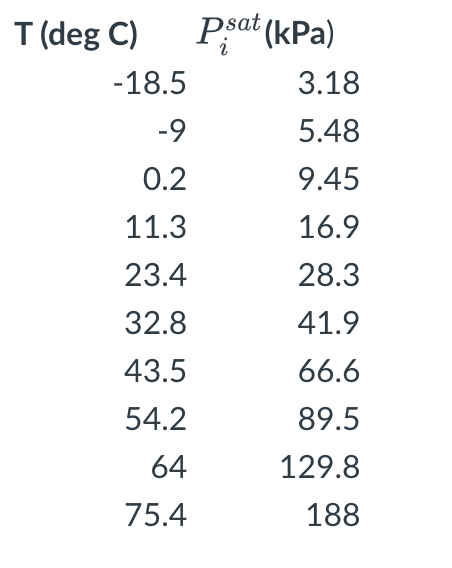
Question: The saturation pressures for an unknown compound A has been measured at several different temperatures (see Table 1 below). (1 point) What is the normal
The saturation pressures for an unknown compound A has been measured at several different temperatures (see Table 1 below).
(1 point) What is the normal boiling point (at 1 bar pressure)? Hint: you will need to fit the data to an Antoine equation.
(2 points) Assume that compound A is now mixed with water. Draw the Pxy diagram at T = 25C and identify the 2 phase region.
(2 points) Draw a Txy diagram at 5 bar pressure.
Solve using Python
T(degC)18.590.211.323.432.843.554.26475.4Pisat(kPa)3.185.489.4516.928.341.966.689.5129.8188
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


