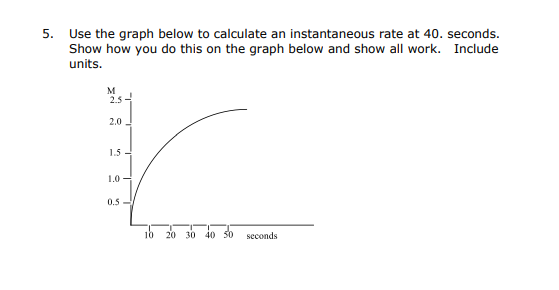
Question: Please answer all, thank you so much! Use the graph below to calculate an instantaneous rate at 40 . seconds. Show how you do this
 Please answer all, thank you so much!
Please answer all, thank you so much!
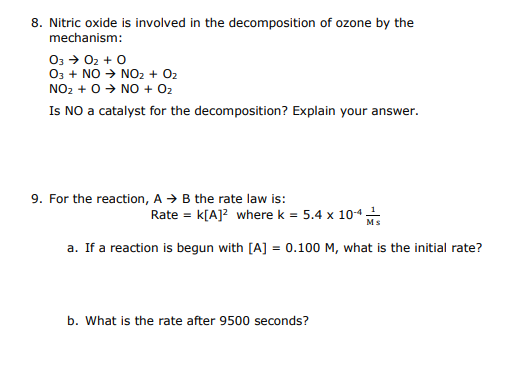
Use the graph below to calculate an instantaneous rate at 40 . seconds. Show how you do this on the graph below and show all work. Include units. 8. Nitric oxide is involved in the decomposition of ozone by the mechanism: O3O2+OO3+NONO2+O2NO2+ONO+O2 Is NO a catalyst for the decomposition? Explain your answer. 9. For the reaction, AB the rate law is: Rate=k[A]2wherek=5.4104Ms1 a. If a reaction is begun with [A]=0.100M, what is the initial rate? b. What is the rate after 9500 seconds
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


