Question: Please answer all the questions. thank you so much. 6. The electron configuration of indium is [Kr]4d105s25p1. Indium forms compounds with chlorine. One is indium

![electron configuration of indium is [Kr]4d105s25p1. Indium forms compounds with chlorine. One](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8e5f37963a_82766f8e5f3212ea.jpg)

![of +2. The electronic configuration of zinc is [Ar] 3d104s2, which is](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8e5f5eab7d_82966f8e5f58e5a3.jpg)
![also written [Ar] 4s2 3d10. From which orbital does zinc lose electrons](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8e5f68e3e2_83066f8e5f62d7f0.jpg)

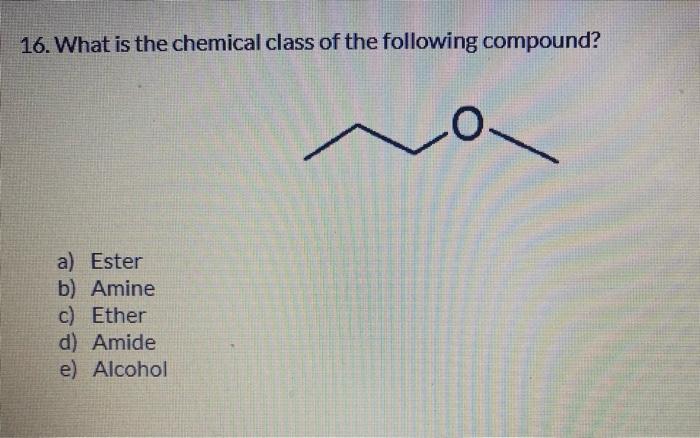
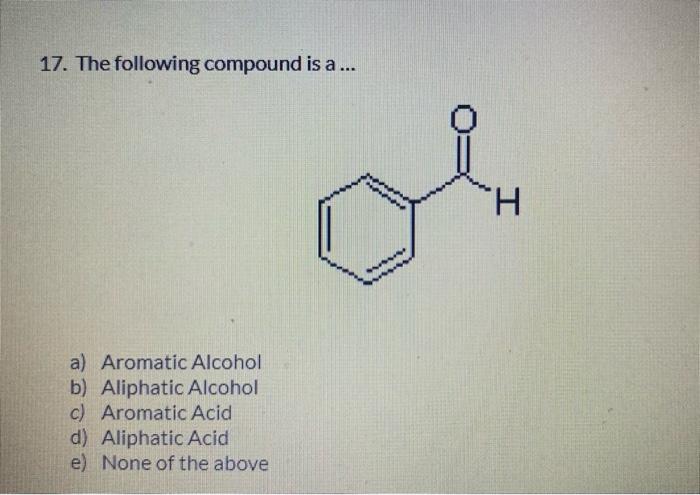
6. The electron configuration of indium is [Kr]4d105s25p1. Indium forms compounds with chlorine. One is indium (I) chloride, formula InCl. What electron do you think indium gave up? a) 4d10 b) 5s2 c) 5p1 d) 4s2 e) 4p1 7. When zinc combines with non-metals, it always has an oxidation number of +2. The electronic configuration of zinc is [Ar] 3d104s2, which is also written [Ar] 4s2 3d10. From which orbital does zinc lose electrons when it form s an ionic bond? a) 4s2 b) 3s2 c) 3d10 d) 2d10 e) 3p6 8. Which of the following is the correct formula for the equilibrium constant of the following reaction? 2SO3()2SO2())+O2()) a) Keq=[SO3]2/[SO2]2[O2] b) Keq=[SO3]2/[SO2]2+[O2] c) Keq=[SO2]2[OO2]/[SO3]2 d) Keq=[SO2]2+[O2]/[SO3]2 e) Keq=[SO2]2[O2]/[SO3]1 14. What is the chemical class of the following compound? CH3(CH2)2CH(CH3)COOH a) Alkane b) Cycloalkane c) Aldehyde d) Carboxylic Acid e) Ketone 15. 3-pentanol \& 2-ethyl butanol are isomers. The previous statement is ... a) True b) False 16. What is the chemical class of the following compound? a) Ester b) Amine c) Ether d) Amide e) Alcohol 17. The following compound is a ... a) Aromatic Alcohol b) Aliphatic Alcohol c) Aromatic Acid d) Aliphatic Acid e) None of the above 18. The type of polymerization in the following reaction is ... polymerization. HO a) Condensation b) Addition c) Elimination d) Isomerization e) Aromaticity 19. An amine is characterized by which functional group? a) NO2 b) NO2 c) NH2 d) =NOH 21. A substance needs more energy to undergo an increase of 5C, this substance has ... specific heat. a) High b) Low c) Moderate d) None of the above e) All of the above 22. The addition of 250.0J to 30.0g of copper, initially at 22C, will change its temperature to what final value? (Cc=0.387sg11C1) a) 30C b) 41C c) 52C d) 43.5C e) None of the above 31. Consider the following unbalanced redox reaction: ClO31+Fe+2+H+1Cl1+Fe+3+H2O The coefficients in the balanced equation are, from left to right: a) 1,1,6,1,1,3 b) 1,2,6,1,2,3 c) 2,3,12,2,3,6 d) 1,6,6,1,6,3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


