Question: please answer all three i will rate. answer all plllllllz What is the base pKa value for the following molecule's most acidic proton? TIP: Base
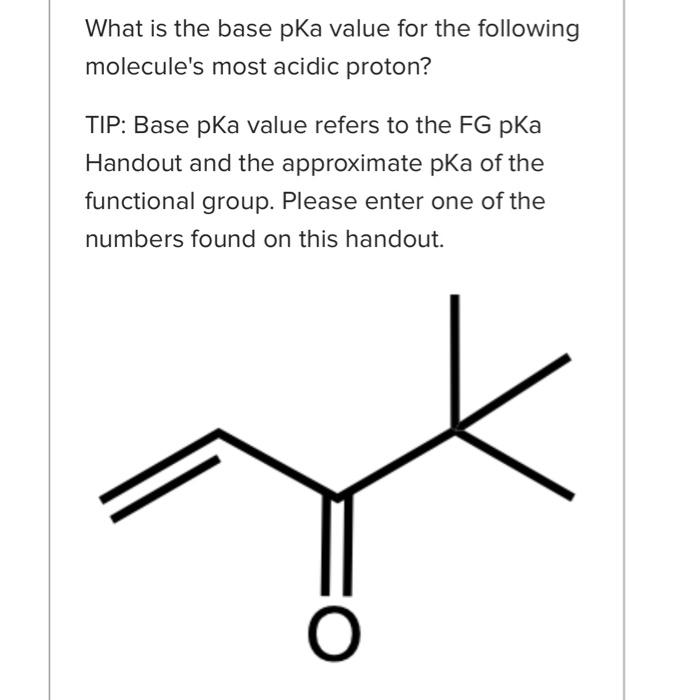
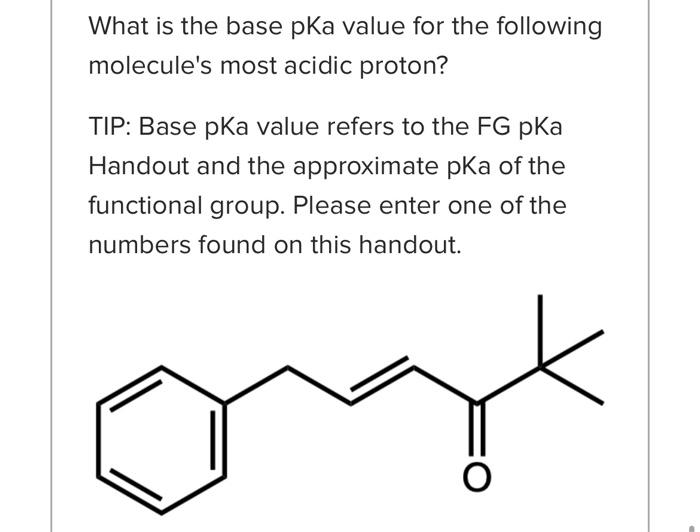
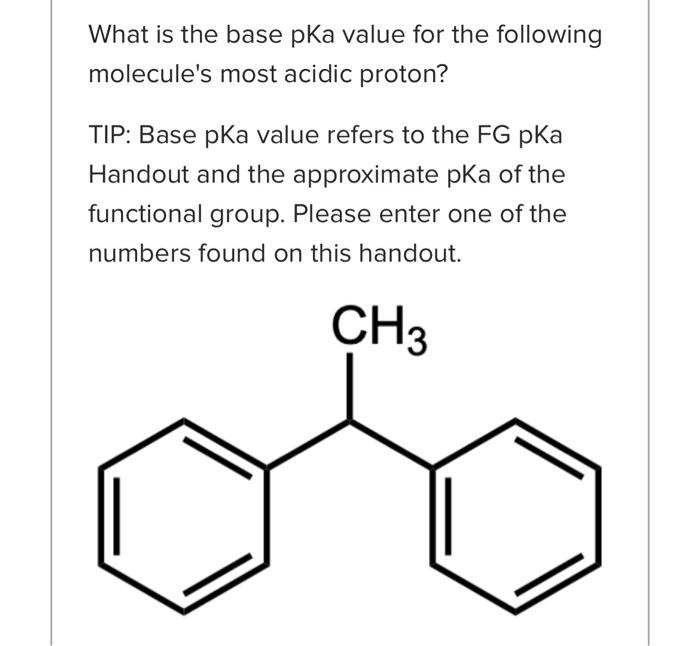
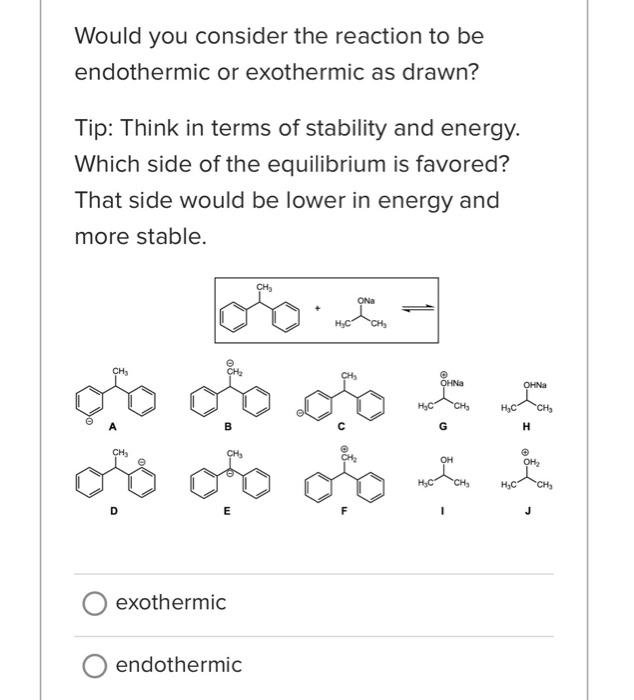
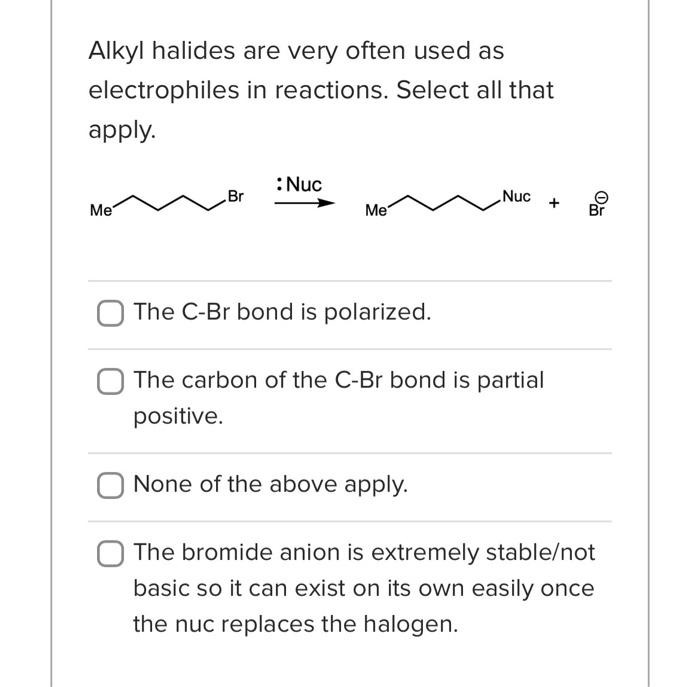
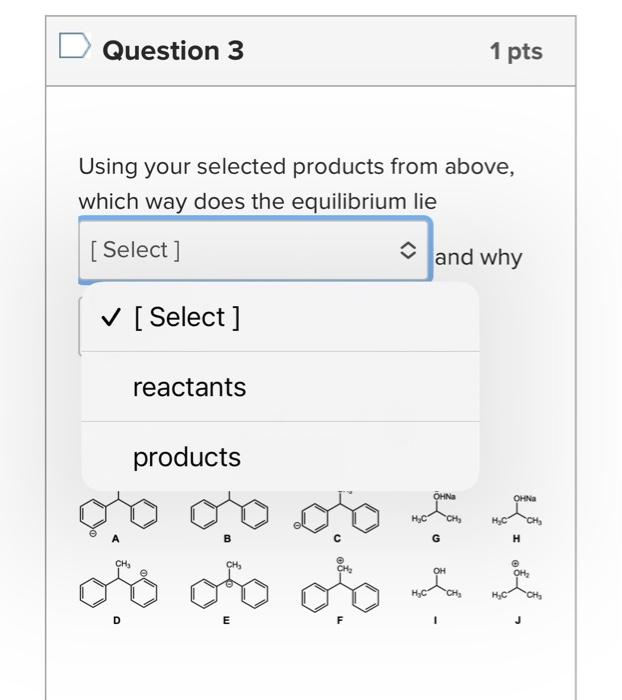
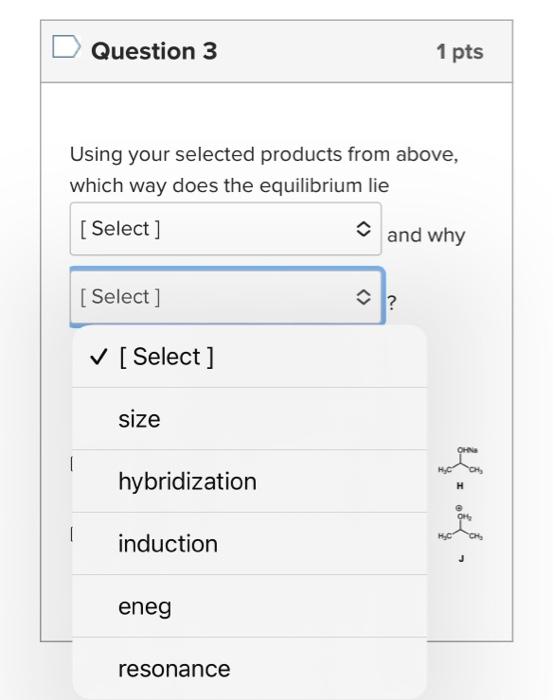
What is the base pKa value for the following molecule's most acidic proton? TIP: Base pKa value refers to the FG pKa Handout and the approximate pKa of the functional group. Please enter one of the numbers found on this handout. What is the base pKa value for the following molecule's most acidic proton? TIP: Base pKa value refers to the FG pKa Handout and the approximate pKa of the functional group. Please enter one of the numbers found on this handout. What is the base pKa value for the following molecule's most acidic proton? TIP: Base pKa value refers to the FG pKa Handout and the approximate pKa of the functional group. Please enter one of the numbers found on this handout. Would you consider the reaction to be endothermic or exothermic as drawn? Tip: Think in terms of stability and energy. Which side of the equilibrium is favored? That side would be lower in energy and more stable. exothermic endothermic Alkyl halides are very often used as electrophiles in reactions. Select all that apply. The CBr bond is polarized. The carbon of the CBr bond is partial positive. None of the above apply. The bromide anion is extremely stableot basic so it can exist on its own easily once the nuc replaces the halogen. Question 3 1 pts Using your selected products from above, which way does the equilibrium lie and why products Question 3 1 pts Using your selected products from above, which way does the equilibrium lie and why ? hybridization induction eneg resonance
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


