Question: please answer all Use the References ta acress important values if needed for this noestion. A helium-filled weather balloon has a volume of 803L at
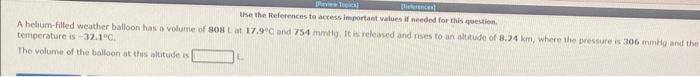


Use the References ta acress important values if needed for this noestion. A helium-filled weather balloon has a volume of 803L at 17.9C and 754mm thg it is releared and nses to an altitude of 8.24 km, where the gressure is a06 mmelg and the temperature is 321C. The volume of the balloon at this alutude is A sample of nitrogen gas occupies a volurne of 9.12Lat48.0C and 0.710atm. If ic is desired to increase the volume of the gas sample to 11.5L, while increasing its prossure to 0.938 atm, the temperature of the gas sample at the new volume and pressure must be aC. A sample of krypton gas occupies a volume of 6.05L it 55.0C and 391 torr. If the volume of the gas sample in decreased to 3.52.4, while its temperature is decreased to 4.000, the fissultinig gas pressure will be
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


