Question: please answer all will be very appreciated 1. Answer the following questions about the reactions given in the table below: 1 . 2 x 104
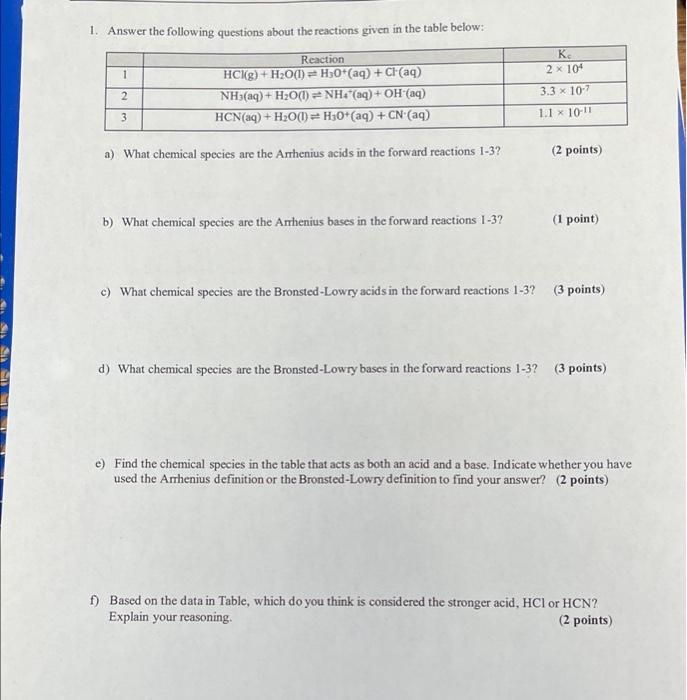

1. Answer the following questions about the reactions given in the table below: 1 . 2 x 104 3.3 x 10-7 1.1 x 10-11 Reaction HCKg) + H2O(1) H3O+ (aq) + Cl(aq) NH3 (aq) + H2O(1) = NH4(aq) + OH (aq) HCN (aq) + H2O(1) - H3O+ (aq) + CN (aq) 2 3 + a) What chemical species are the Arrhenius acids in the forward reactions 1-3? (2 points) b) What chemical species are the Arrhenius bases in the forward reactions 1-3? (1 point) c) What chemical species are the Bronsted-Lowry acids in the forward reactions 1-3? (3 points) d) What chemical species are the Bronsted-Lowry bases in the forward reactions 1-3? (3 points) e) Find the chemical species in the table that acts as both an acid and a base. Indicate whether you have used the Arrhenius definition or the Bronsted-Lowry definition to find your answer? (2 points) f) Based on the data in Table, which do you think is considered the stronger acid, HCl or HCN? Explain your reasoning. (2 points) 2. Consider reaction 1 2) What species results from the loss of a proton from the Bronsted-Lowry acid in the forward reaction? (1 point) b) Does the species indicated in partn) (the answer that you gave) act as an acid or base when the reverse of reaction 1 occurs? (1 point) c) What species results from the gain of a proton by the Bronsted-Lowry base in the forward reaction? (1 point) d) Does the species indicated in part c) act as an acid or a base when the reverse of reaction 1 occurs? (1 point) e) Answer parts a)-d) for reactions 2 and 3 also. Describe any general relationship that you observe using a grammatically correct sentence. (9 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


