Question: please answer A-N with the info provided asap pleae. Initial reagent masses. 6.08. Mass of NiCl-6H2O Volume of 15 M NH; Mass of purple Ni
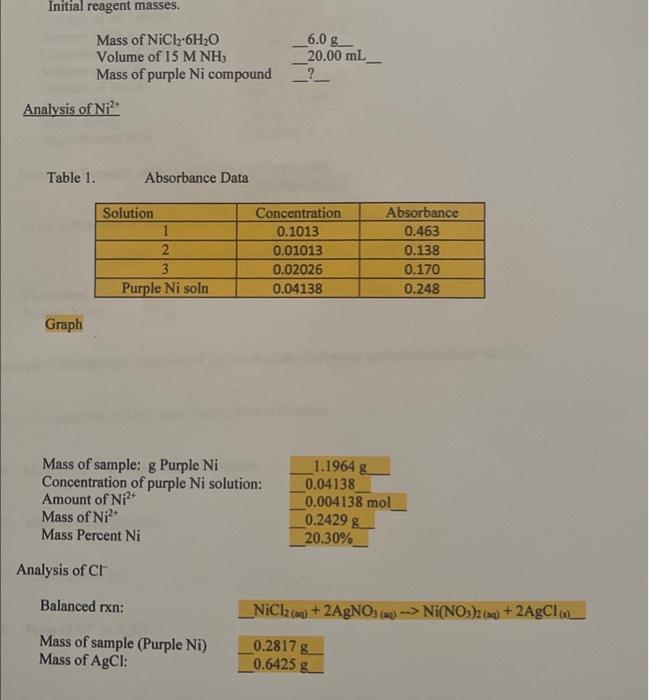



Initial reagent masses. 6.08. Mass of NiCl-6H2O Volume of 15 M NH; Mass of purple Ni compound 20.00 mL ? Analysis of Ni? Table 1. Absorbance Data Solution 1 2 3 Purple Ni soln Concentration 0.1013 0.01013 0.02026 0.04138 Absorbance 0.463 0.138 0.170 0.248 Graph Mass of sample: g Purple Ni Concentration of purple Ni solution: Amount of Ni2+ Mass of Ni? Mass Percent Ni 1.1964 g 0.04138 0.004138 mol _0.2429 g 20.30% Analysis of CT Balanced rxn: _NiCl2(aq) + 2AgNO3 () -> Ni(NO3)2(a) + 2AgCla + Mass of sample (Purple Ni) Mass of AgCl: 0.2817 g 0.6425 g Mass of CI: Mass Percent CI: 0.1589 24.73% Analysis of NH Balanced rxns (both) Mass of sample (Purple Ni): Concentration of HCI Volume of HCI added Amount of HCI Concentration of NaOH Volume of NaOH Amount of NaOH Amount NH) Mass of NH Mass Percent NH HCI + NH3 --> NHClow + HCI NaOH --> NaCI+H70 0.2749g 0.2034 34.88 mL 0.007095 mol 0.1023 3.18 ml 0.000325 mol 0.00677 mol 0.1153 g 41.19% Empirical Formula of the Purple Nickel Compound: _Ni(NH3) Ch. Balance Reaction of the Synthesis of the Purple Nickel Compound Ni(NH) Cb + 6HCI -->NICI + 6NH +6C! Sample Calculations Show work for each of the following calculations from the A. Concentration of Unknown Purple Nickel Solution B. Mole of Ni* in Purple Nickel Solution C. Mass of Ni in Solution D. Mass Percent Ni? E. Mass of CI in AgCl F. Mass Percent CI G. Mole of HCI added H. Mole of NaOH used I. Mole of NH3 J. Mass of NH; K. Mass Percent NH3 L. Empirical Formula determination M. Determination of the Theoretical Yield i. Based on NiCl 6H2O ii. Based on NH3 N. Percent Yield Initial reagent masses. 6.08. Mass of NiCl-6H2O Volume of 15 M NH; Mass of purple Ni compound 20.00 mL ? Analysis of Ni? Table 1. Absorbance Data Solution 1 2 3 Purple Ni soln Concentration 0.1013 0.01013 0.02026 0.04138 Absorbance 0.463 0.138 0.170 0.248 Graph Mass of sample: g Purple Ni Concentration of purple Ni solution: Amount of Ni2+ Mass of Ni? Mass Percent Ni 1.1964 g 0.04138 0.004138 mol _0.2429 g 20.30% Analysis of CT Balanced rxn: _NiCl2(aq) + 2AgNO3 () -> Ni(NO3)2(a) + 2AgCla + Mass of sample (Purple Ni) Mass of AgCl: 0.2817 g 0.6425 g Mass of CI: Mass Percent CI: 0.1589 24.73% Analysis of NH Balanced rxns (both) Mass of sample (Purple Ni): Concentration of HCI Volume of HCI added Amount of HCI Concentration of NaOH Volume of NaOH Amount of NaOH Amount NH) Mass of NH Mass Percent NH HCI + NH3 --> NHClow + HCI NaOH --> NaCI+H70 0.2749g 0.2034 34.88 mL 0.007095 mol 0.1023 3.18 ml 0.000325 mol 0.00677 mol 0.1153 g 41.19% Empirical Formula of the Purple Nickel Compound: _Ni(NH3) Ch. Balance Reaction of the Synthesis of the Purple Nickel Compound Ni(NH) Cb + 6HCI -->NICI + 6NH +6C! Sample Calculations Show work for each of the following calculations from the A. Concentration of Unknown Purple Nickel Solution B. Mole of Ni* in Purple Nickel Solution C. Mass of Ni in Solution D. Mass Percent Ni? E. Mass of CI in AgCl F. Mass Percent CI G. Mole of HCI added H. Mole of NaOH used I. Mole of NH3 J. Mass of NH; K. Mass Percent NH3 L. Empirical Formula determination M. Determination of the Theoretical Yield i. Based on NiCl 6H2O ii. Based on NH3 N. Percent Yield
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


