Question: Please answer and explain step by step questions 21,22 and 23. Use dimensional analysis and the density of silver, 13.37g/cm3 to calculate the volume (in
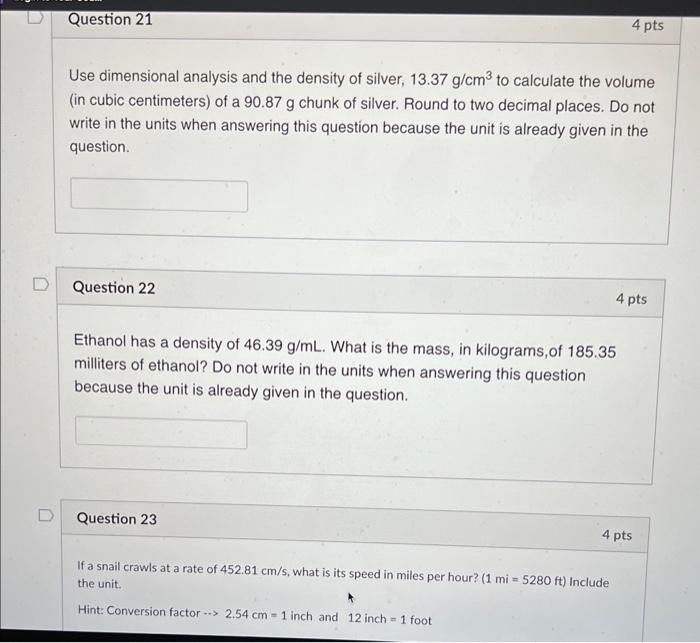
Use dimensional analysis and the density of silver, 13.37g/cm3 to calculate the volume (in cubic centimeters) of a 90.87g chunk of silver. Round to two decimal places. Do not write in the units when answering this question because the unit is already given in the question. Question 22 4 pts Ethanol has a density of 46.39g/mL. What is the mass, in kilograms, of 185.35 milliters of ethanol? Do not write in the units when answering this question because the unit is already given in the question. Question 23 If a snail crawls at a rate of 452.81cm/s, what is its speed in miles per hour? (1mi=5280ft) include the unit. Hint: Conversion factor 2.54cm=1 inch and 12 inch =1 foot
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


