Question: please answer and I will upvote A mixture consisting of methyl-propane, n-butane, n-pentane, 2-methyl-pentane, and nhexane is to be separated via continuous distillation. The feed

please answer and I will upvote
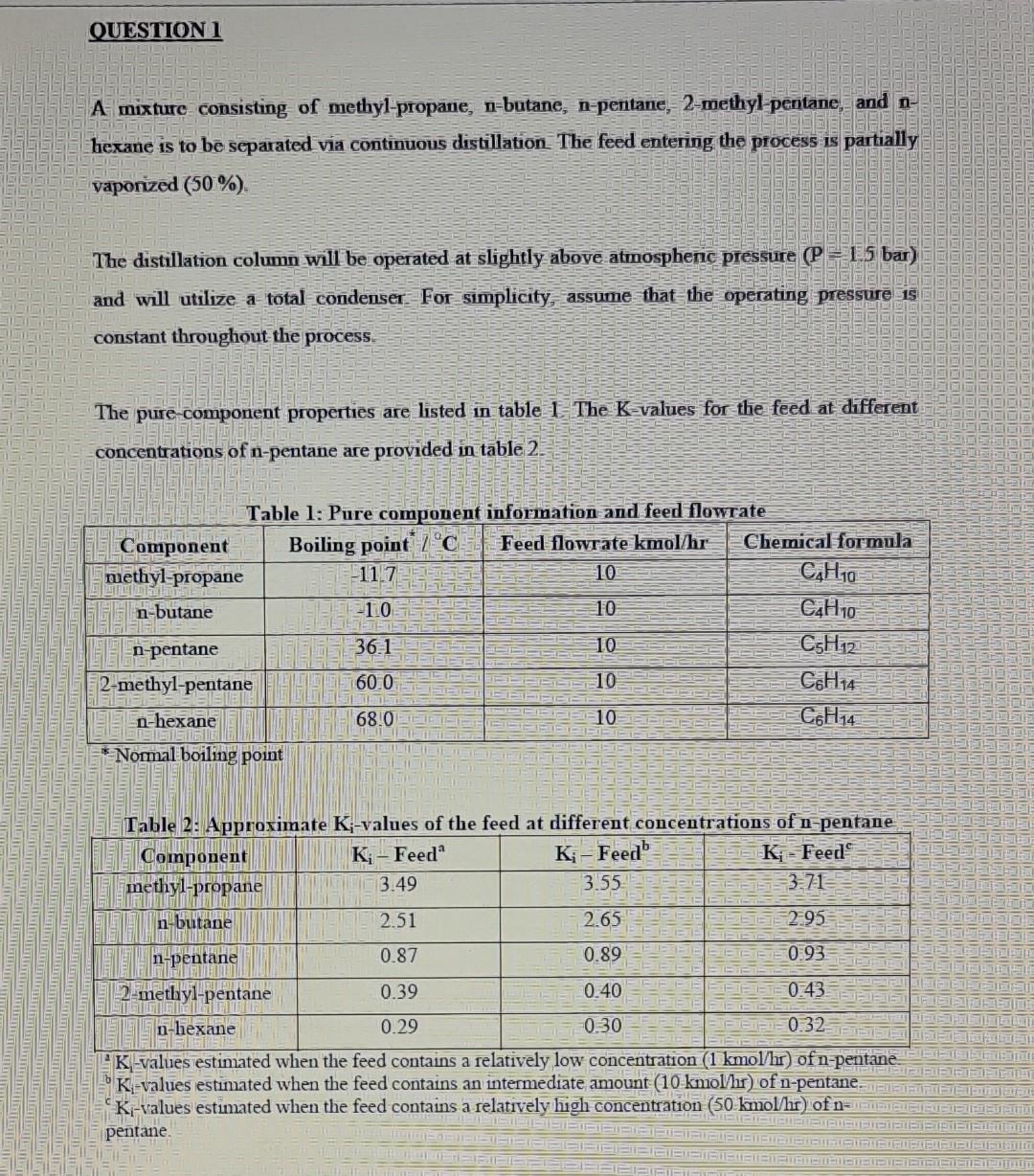
A mixture consisting of methyl-propane, n-butane, n-pentane, 2-methyl-pentane, and nhexane is to be separated via continuous distillation. The feed entering the process is partially vaponized (50%) The distillation column will be operated at slightly above atmosphenc pressure ( P=1.5 bar) and will utilize a total condenser For simplicity, assume that the operating pressure is constant throughout the process. The pure-component properties are listed in table I - The K-values for the feed at different concentrations of n-pentane are provided in table 2 . Table 2. Annnoximate K :-values of the feed at different concentrations of n-pentane 2K-values estimated when the feed contains a relatively low concentration ( 1kmo/hr) of n-pentane Kl-values estimated when the feed contains an intermediate amount (10kmol/hr) of n-pentane. cK1-values estimated when the feed contains a relatively high concentration ( 50kmol/hr) of n pentane. 1.1. Given the feed information in table 1, suppose that methyl-propane and n-butane are to be separated from 2-methyl-pentane and n-hexane using a single continuous distillation column. Approximate the minimum number of theoretical trays for this column, assuming that there is an equimolar split of n-pentane, and given a recovery of 99.9% for both n-butane and 2-methyl-pentane. [10] 1.2. Calculate the distribution of the two non-key components directly adjacent to the key components. [5] 1.3. Provide a solution strategy to estimate the minimum reflux ratio for this distillation column (only the method is required, not the actual value for the minimum reflux). [4] 4. Explain how the Gilliland Correlation was developed, furthermore comment on its applicability and accuracy in today's age with the advent of the modern process simulator
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


