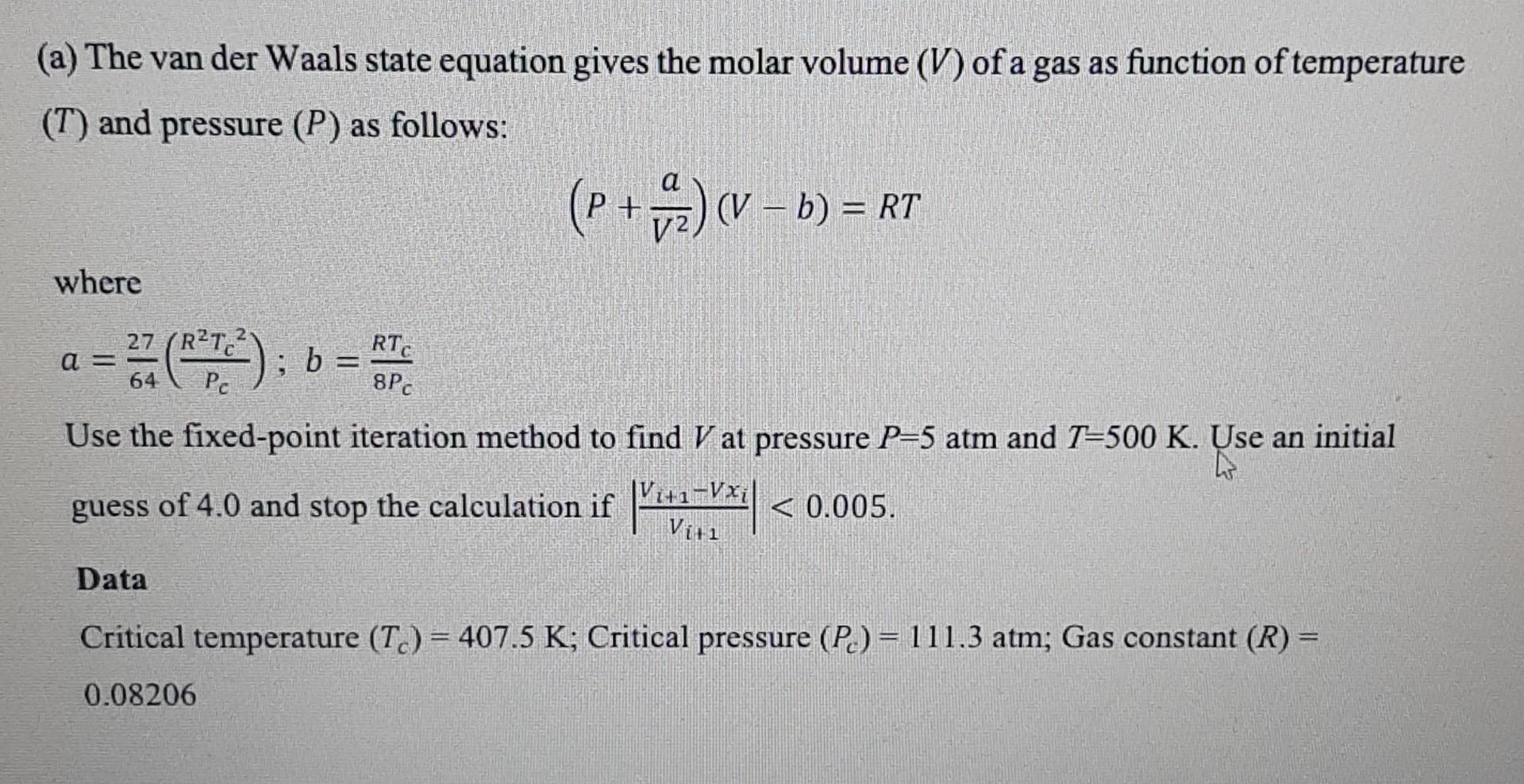
Question: please answer and justify the answer (a) The van der Waals state equation gives the molar volume (V) of a gas as function of temperature
please answer and justify the answer
(a) The van der Waals state equation gives the molar volume (V) of a gas as function of temperature (T) and pressure (P) as follows: (P+V2a)(Vb)=RT where a=6427(PcR2Tc2);b=8PcRTc Use the fixed-point iteration method to find V at pressure P=5atm and T=500K. Use an initial guess of 4.0 and stop the calculation if vi+1Vi+1Vxi
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


